At-Home
Remote Management of Pacemaker Patients with Biennial In-clinic evaluation: Continuous Home Monitoring in the Japanese at Home Study. A Randomized Clinical Trial
Watanabe E ET AL., CIRCEP 2020 May;13(5):e007734
doi: 10.1161/CIRCEP.119.007734
Study Design
- Large, prospective, randomized, controlled, multicenter study
- 1274 consecutive pacemaker patients at 85 centers in Japan
- Single- or dual chamber pacemaker indication according to Japanese guidelines
- BIOTRONIK Evia devices
- 1:1 randomization to Home Monitoring+remote follow-up or Home Monitoring+in-office follow-up
- Regular biannual follow-ups in both arms
- If needed, unscheduled in-office follow-ups initiated by patients or physicians or due to alerts notified by HM
- 24 months follow-up duration
Key Result 1
The At-Home study demonstrated non-inferiority of exclusive remote follow-up over 2 years in comparison to continuous remote monitoring associated with in-office follow-ups for real-world pacemaker patients.
Figure 1: Kaplan-Meier curves of incidence of primary endpoints (a composite of death, stroke, or cardiovascular surgical procedure), starting at randomization. RFU, remote follow-up; CFU, conventional follow-up.

Key Result 2
The median (IQR) number of in-office follow-ups was 0.50 (0.50 – 0.63) in RFU and 2.01 (1.93 – 2.05) in CFU per patient-year (P<0.001).

Clinical Relevance
- The At-Home study is the largest randomized trial to investigate pacemaker patient follow-up with Home Monitoring only for 2 years.
- Follow-up of cardiovascular implantable electronic devices is the most frequent activity reported by cardiac electrophysiologists. By exclusively using Home Monitoring, the proportion of in-office follow-ups can be significantly reduced for pacemaker patients.
- Before At-Home, the main concern about extending the time between in-office follow-ups was that safety could be compromised. The At-Home results indicate that surveillance of a real-world pacemaker patient population exclusively based on Home Monitoring for 2 years is safe.
- The At-Home results indicate that reducing the need for in-office follow-ups reduces the resource consumption of managing pacemaker patients.
| Study Objective |
|
|---|---|
| Primary Endpoint |
|
| Major Secondary Endpoints |
|
| Clinical Sites |
|
| Sample Size |
|
| Main Inclusion Criteria |
|
| Main Exclusion Criteria |
|
| Study Flowchart | 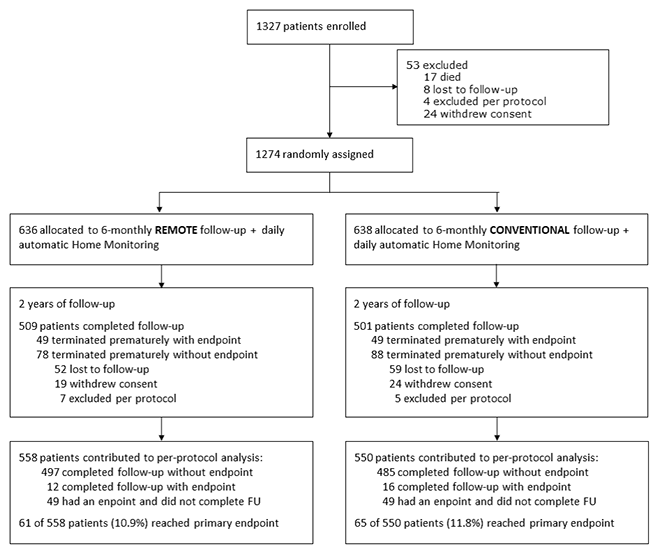 |
| Follow-Up |
|
| Study Duration |
|
| Reference no. |
|
| Principal Investigators |
|
