BIOFLEX-I
NCT01319812
Prospective, international, multi-center, investigational device exemption trial evaluating BIOTRONIK Astron nitinol self-expanding stent for iliac arteries
Conclusion
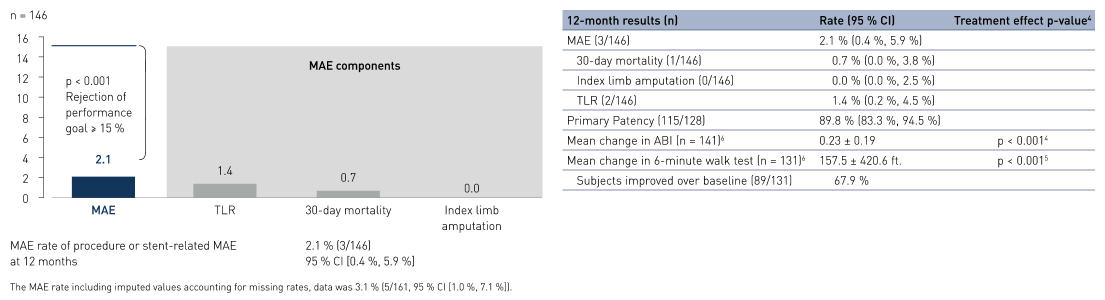
- At 12 months results show primary patency (PP) rates of 89.8% (115/1281 patients) and freedom from Target Lesion Revascularization (FTLR) rates of 98.6% (144/146 2 patients).
- At 12 months, major adverse event (MAE) rate is 2.1%, showing safety of the treatment and meeting the primary endpoint.
- Improvement in ankle brachial index (ABI) shows clinical benefit with mean change from baseline to 12 months of 0.23 ± 0.19.
- The Astron stent is a viable option for the treatment of patients with iliac artery disease.
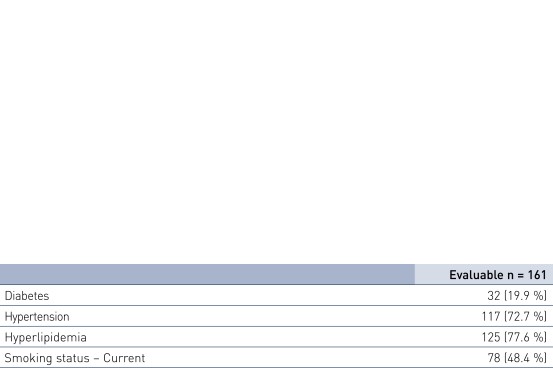
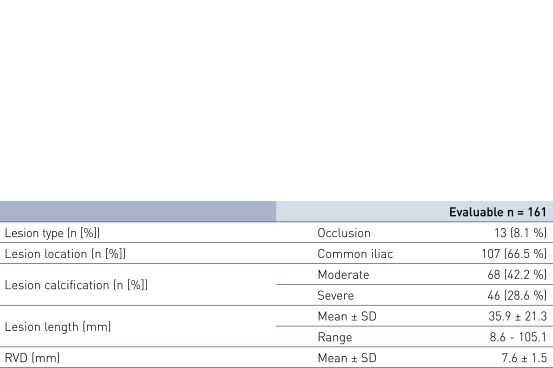
Patient Demographics and Lesion Characteristics
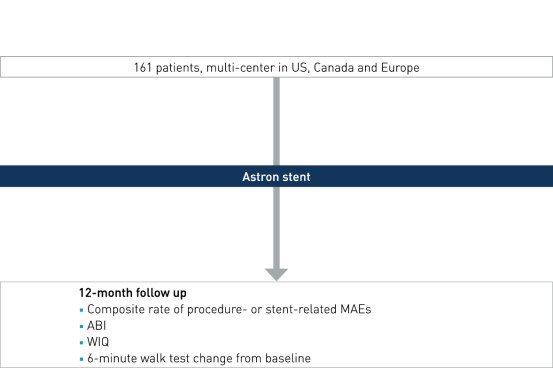
Study Design
Prospective, international, multi-center, investigational device exemption trial evaluating BIOTRONIK Astron nitinol self-expanding stent for iliac arteries, conducted at 30 centers in the US, Canada and Europe.
- Number of patients (n): 161
- Principal investigators: Dr. Mark Burket, University of Toledo, Ohio, United States, and Dr. Marianne Brodmann, University of Graz, Austria
- Primary endpoint: composite rate of procedure- or stent-related MAEs at 12 months, post-index procedure (30-day mortality, 12-month clinically-driven TLR and index-limb amputation)
- Secondary clinical endpoints (selected): components of MAE, PP at 12 months assessed by DUS (PSVR > 2.4) 3, acute procedural success, ankle-brachial index (ABI), walking impairment questionnaire (WIQ) and 6-minute walk test change from baseline to 12 months
Primary Endpoint Results
MAE rate composite and components at 12 months
Downloads

Vascular Intervention
Self-Expanding StentPull-back delivery system for simple stent deployment

Vascular Intervention
Self-Expanding StentS-articulating connecting bars and peak-to-valley design for multi-directional flexibility
Source:
BIOFLEX-I 12m, Burket M. Presented at CRT conference 2015.
1 ITT (intention to treat population) with DUS (duplex ultra sound).
2 ITT completed subjects.
3 Loss of patency defined as or based on a clinically indicated TLR with angiographic evidence of > 50% stenosis.
4 Two-sided test for difference equal to zero
5 Test for difference equal to zero (Student's t-test, two-sided and Wilcoxon Test, two-sided)
6 Subjects with paired data (baseline and 12 months)
© BIOTRONIK AG
All rights reserved. Specifications are subject to modification, revision and improvement.
