BIOFLOW-I
NCT01214148
First in man experience with a drug-eluting stent in de novo coronary artery lesions
Conclusion
- In BIOFLOW-I, the Orsiro hybrid DES showed excellent Late Lumen Loss (LLL) results in the overall patient population.
- BIOFLOW-I has a high rate of diabetic patients and complex lesions, atypical for a FIM study.
- There was no late catch-up in LLL values at 9-month follow-up. This combined with a narrow standard deviation suggests robust study results.
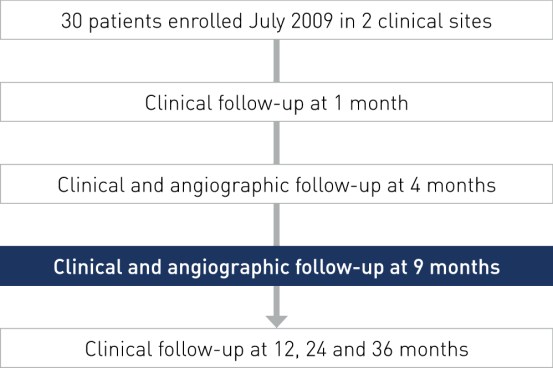
Study Design
- Prospective, multi-center, non-randomized, first-in-man trial
- Primary endpoint: In-stent late lumen loss at 9 months by QCA
- Coordinating clinical investigator: Prof. Martial Hamon, University Hospital of Caen, France
- Principal investigators: Dr. Rodica Niculescu and Dr. Dan Deleanu
- Objective: To assess the safety and clinical performance of the Orsiro hybrid drug-eluting stent in patients with single de novo coronary artery lesions
Primary endpoint
Cumulative frequency of in-stent Late Lumen Loss at 4 and 9 months

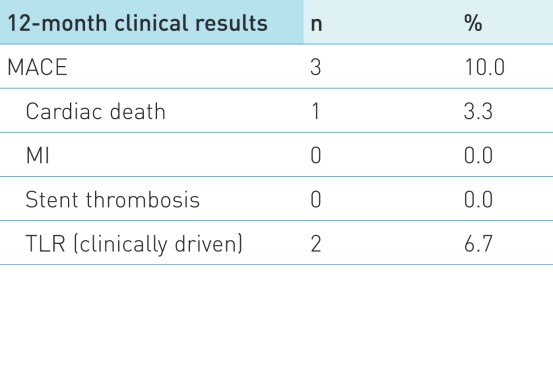
Secondary endpoint
MACE defined as composite of cardiac death, MI attributed to the target vessel, stent thrombosis and clinically driven target lesion revascularization. Stent thrombosis was defined according to the Academic Research Consortium definitions.
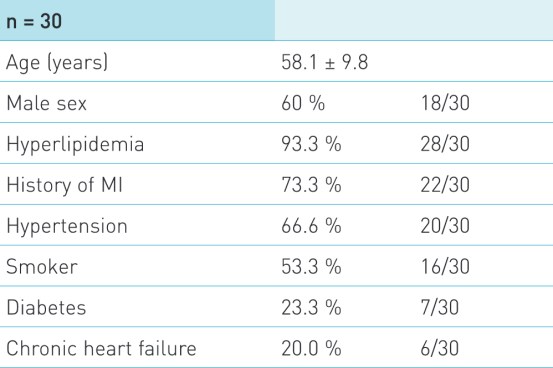
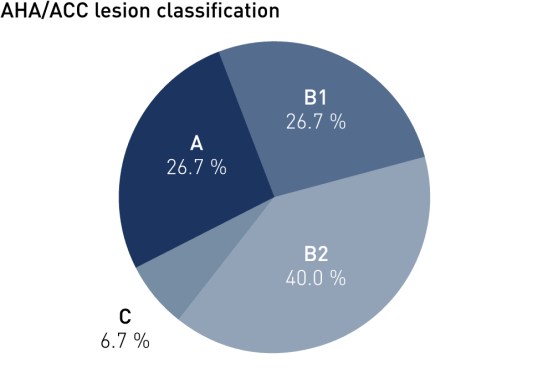
Baseline Characteristics
Downloads

Source
Hamon M et al. Clinical and angiographic experience with a third-generation drug-eluting Orsiro stent in the treatment of single de novo coronary artery lesions (BIOFLOW-I): a prospective, first-in-man study. EuroIntervention. 2013; 8: 1006-1011.
Disclaimer
© BIOTRONIK AG All rights reserved. Specifications are subject to modification, revision and improvement.
