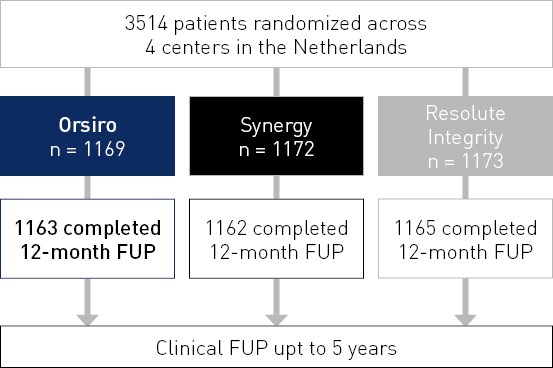
BIO-RESORT RCT
NCT01674803
Comparison of biodegradable polymer and durable polymer drug-eluting stents in an all comers population
Conclusion
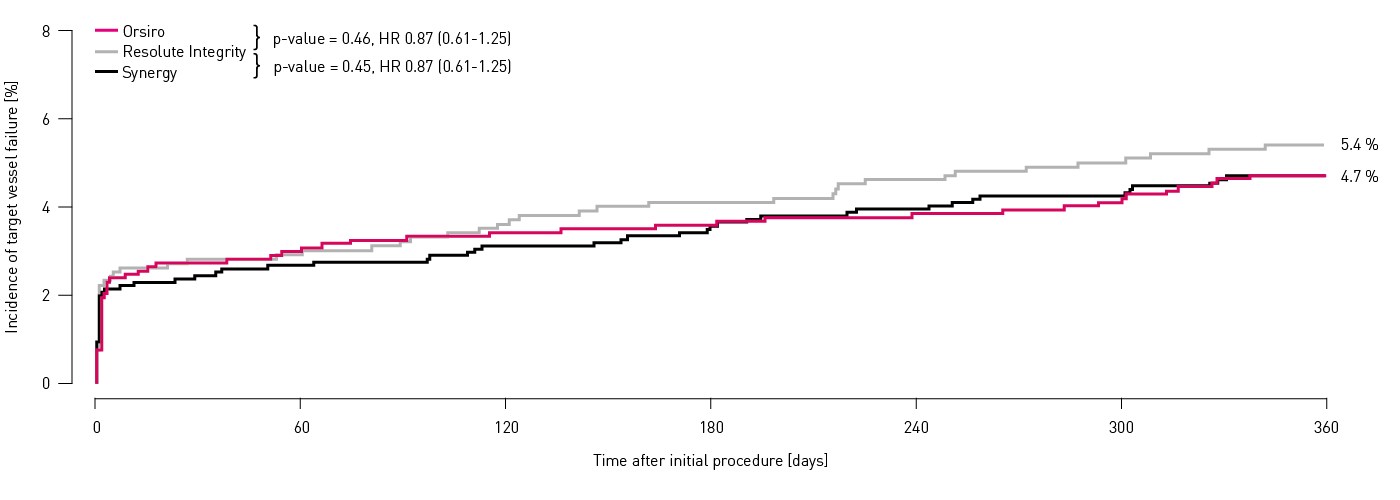
- In this 3514 patient large, randomized, investigator-initiated, all-comers trial, Orsiro proves non-inferiority to Resolute Integrity whilst performing equally well as Synergy (primary endpoint TVF at 12 months: Orsiro 4.7%, Synergy 4.7%, Resolute Integrity 5.4%, p non-inferiority < 0.0001)
- Orsiro, with its ultrathin struts and bioabsorbable polymer, additionally showed safety with a definite stent thrombosis rate of 0.3 % at 12 months, which was equally low in the other study arms.
- These convincing results reconfirm those of previous Orsiro trials and adds to the solid foundation of clinical evidence that supports the use of Orsiro across a broad range of applications.
Study Design
- All-comers, multi-center, assessor and patient-blinded, randomized, non-inferiority design
- Principal Investigator: Prof. Clemens von Birgelen, Enschede, Netherlands
- Primary endpoint: Target Vessel Failure (TVF) at 12 months defined as the composite of cardiac death, target vessel-related Myocardial Infarction (MI), or Target Vessel Revascularization (TVR)
- Prespecified secondary endpoints: Components of the primary endpoint, All-cause mortality, Any Myocardial Infarction, Clinically indicated TLR, Stent thrombosis
Primary endpoint results
Target vessel failure1,2
Downloads

Disclaimer
© BIOTRONIK AG – All rights reserved. Specifications are subject to modification, revision and improvement.
1 von Birgelen C et al. BIO-RESORT (TWENTE III). A Prospective, Randomized Three-Arm Trial Comparing Orsiro, Synergy and Resolute Integrity in an All-Comers Population. TCT 2016. Oral presentation.
2 von Birgelen C et al. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet 2016. Online publication (10.1016. S0140-6736(16); 31920-1).
