CASTLE-AF
Catheter Ablation Versus Standard Conventional Treatment in Patients with Left Ventricular Dysfunction and Atrial Fibrillation
Marrouche NF, Brachmann J et al., New England Journal of Medicine 2018
Study Design
- Prospective, randomized, multicenter, international
- Evaluated the effectiveness of catheter ablation of atrial fibrillation in patients with heart failure on mortality and morbidity when compared to medical treatment
- 398 patients at 33 sites in Europe, USA, and Australia
Key Result 1
Catheter ablation of atrial fibrillation in patients with heart failure is associated with a significant 38% reduction in death or hospitalization for worsening heart failure.
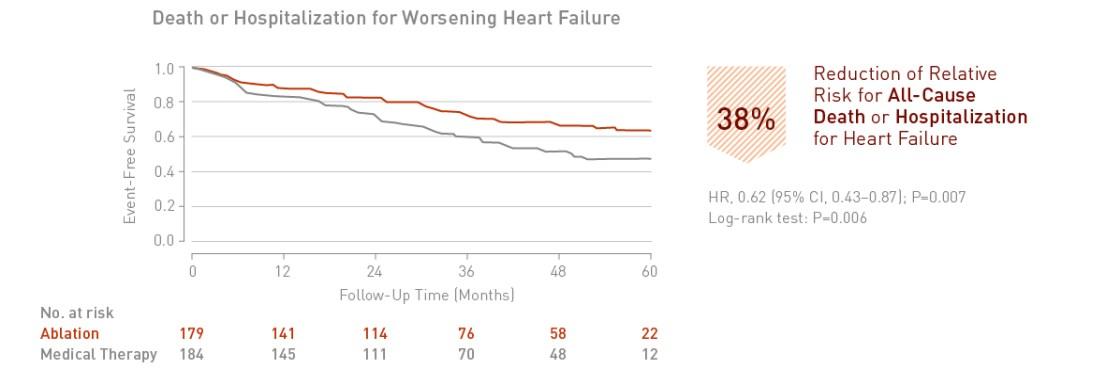
Key Result 2
Catheter ablation of atrial fibrillation in patients with heart failure is associated with a significant 47% reduction in death from any cause.
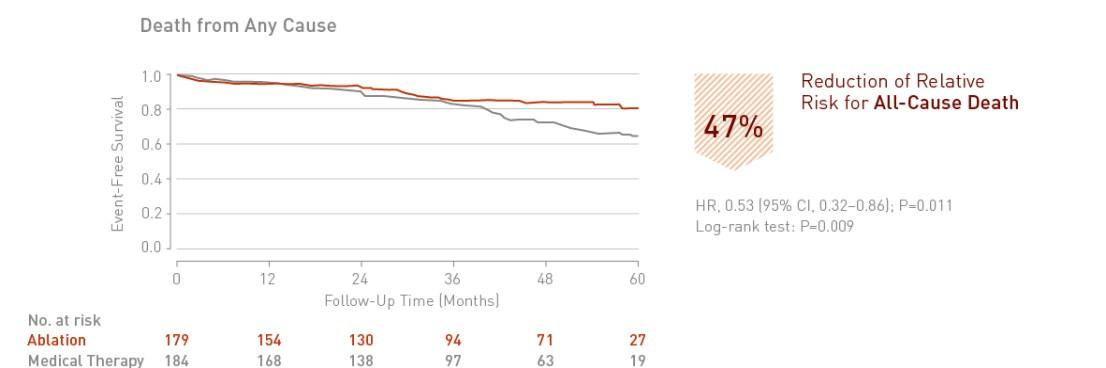
Key Result 3
Catheter ablation of atrial fibrillation in patients with heart failure is associated with a significant 44% reduction in hospitalization for worsening heart failure.
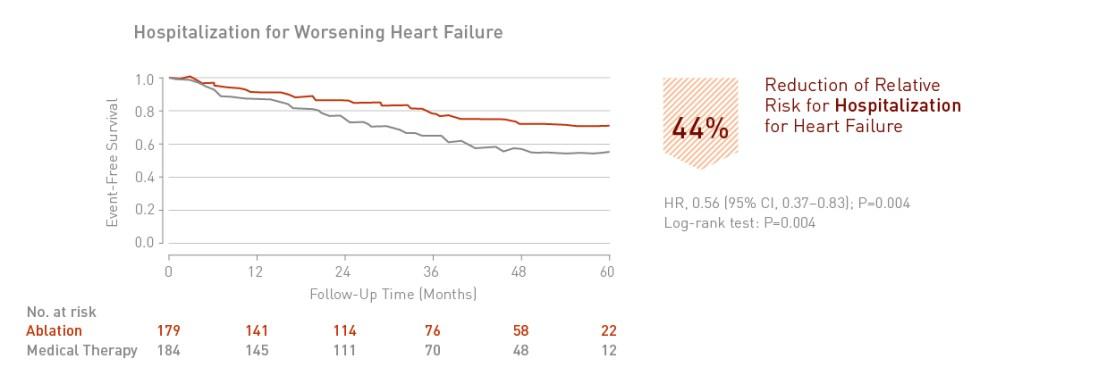
Clinical Relevance
- CASTLE-AF is the first large, randomized study providing clinical evidence that ablation of atrial fibrillation improves hard outcome parameters in heart failure patients
- Catheter ablation for patients with heart failure and concomitant AF can be suggested as a first-line therapy, as early as possible during the course of heart failure
- The results strongly indicate that catheter ablation of atrial fibrillation is a crucial element in managing advanced heart failure, next to CRT and continuous remote monitoring
| Study Objective |
|
|---|---|
| Primary Endpoint |
|
| Major Secondary Endpoints |
|
| Clinical Sites |
|
| Sample Size |
|
| Main Inclusion Criteria |
|
| Main Exclusion Criteria |
|
| Study Flowchart |
 |
| Follow-Up |
|
| Study Duration |
|
| Reference no. |
|
| Principal Investigators |
|

