COMPAS
A randomized trial of long-term remote monitoring of pacemaker recipients (The COMPAS trial)
MABO P ET AL., EUROPEAN HEART JOURNAL, 2012
Study Design
- Multi-center, prospective, large-scale, randomized clinical trial
- 538 patients in 43 French centers
- To evaluate the effects and determine the benefits of long-term follow-up with BIOTRONIK Home Monitoring® on safety and effectiveness in pacemaker patients
Key Result 1
COMPAS demonstrated a 66% reduction in hospitalizations for atrial arrhythmia and related stroke in the Home Monitoring group.
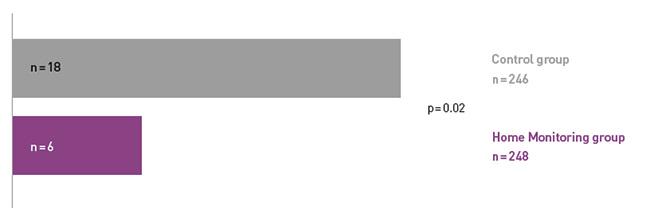
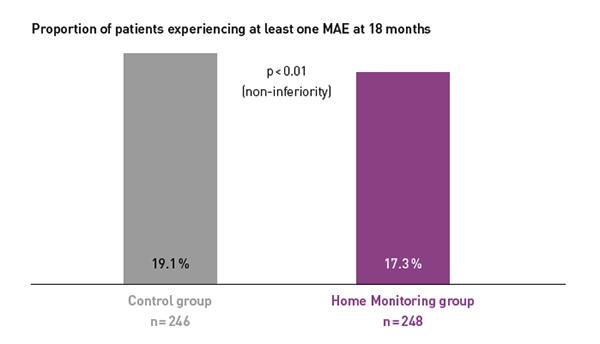
Key Result 2
COMPAS showed comparable safety event rates in both groups.
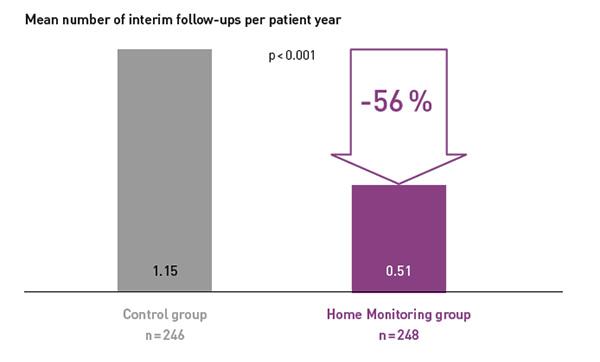
Key Result 3
COMPAS demonstrated that BIOTRONIK Home Monitoring® reduced the number of interim in-office follow-ups by 56 %
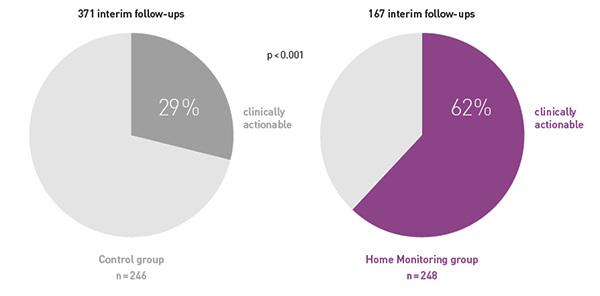
Key Result 4
While at the same time making these follow-ups more often clinically actionable, i.e. resulting in pacemaker reprogramming or medication changes.
Clinical Relevance
- The COMPAS trial results demonstrate that BIOTRONIK Home Monitoring® is a safe and highly effective follow-up method for pacemaker patients.
- BIOTRONIK Home Monitoring® reduces the follow-up burden of overloaded clinics by enabling physicians to focus on patients who are more critically in need of medical attention.
- BIOTRONIK Home Monitoring® enhances patient safety by enabling physicians to intervene earlier in case of a clinically relevant event.
| Study Objective |
|
|---|---|
| Primary Endpoint |
|
| Major Secondary Endpoints |
|
| Clinical Sites |
|
| Sample Size |
|
| Main Inclusion Criteria |
|
| Main Exclusion Criteria |
|
| Study Flowchart |
 |
| Follow-Up |
|
| Study Duration |
|
| Reference no. |
|
| Principal Investigators |
|
