DEBAS Registry
To evaluate the outcome of the implantation of the Pulsar-18 stent followed by Passeo-18 Lux drug-coated balloon in the femoropopliteal arteries
Conclusion
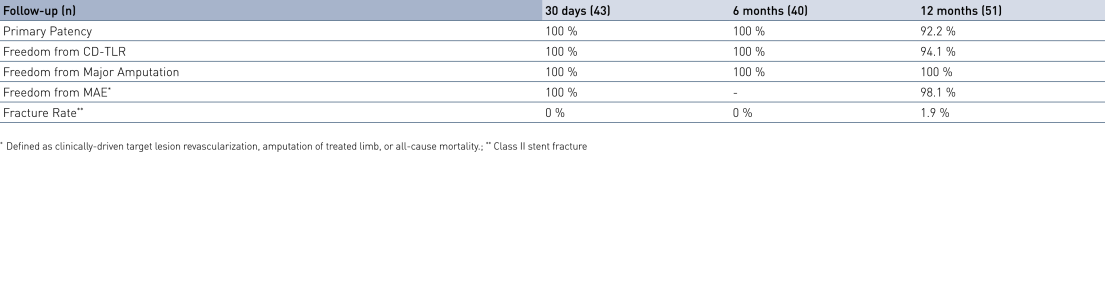
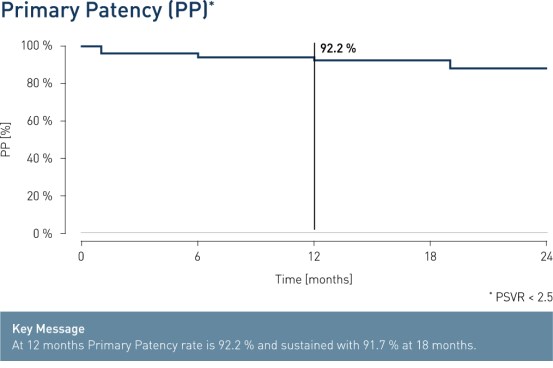
- At 12 months, results from 51 patients show primary patency (PP) rates of 92.2% and freedom from clinically-driven Target Lesion Revascularization (cd-TLR) of 94.1%.
- At 12 months, freedom from major adverse events (MAE) is 98.1%, confirming the safety of the treatment combination with Pulsar-18 and Passeo-18 Lux.
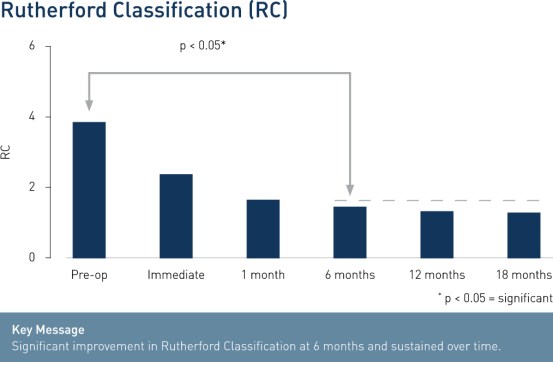
- Significant improvement in Rutherford Class (RC) at 6 months and sustained over time shows clinical benefit and improvement of patients' quality of life.
- The combined approach of Passeo-18 Lux peripheral drug-coated balloon (DCB) and Pulsar-18 self-expanding stent is feasible and promising, and a potential future treatment option in complex, TASC C/D lesions.
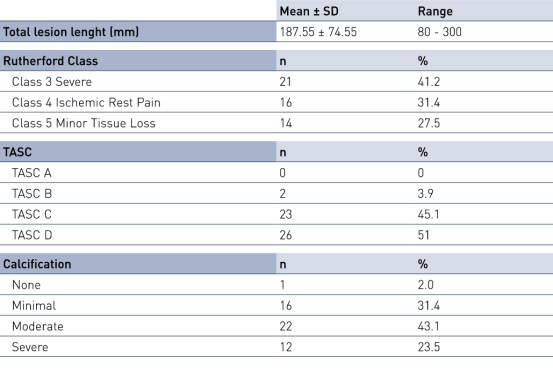
Key Baseline Demographics
Study Design
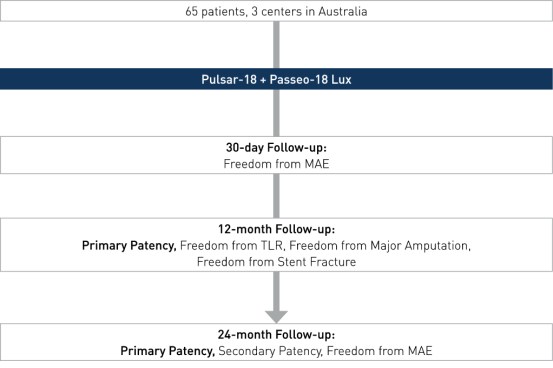
Prospective, multi-center, investigator-initiated registry to evaluate the implantation outcome of the Pulsar-18 stent followed by Passeo-18 Lux DCB in the femoropopliteal arteries.
- Number of patients (n): 65 (12-month data available on n 51)
- Principal investigator: Dr. Patrice Mwipatayi Royal Perth Hospital, University of Western Australia, Perth, Australia
- Primary endpoint: PP at 12 and 24 months, defined as a binary duplex ultrasound ratio PSVR <2.5 at the stented target lesion with no clinically-driven reintervention within the stented segment.
- Secondary clinical endpoints (selected): secondary patency at 12 and 24 months, freedom from MAE at 12 and 24 months, freedom from stent fracture, freedom from TLR, freedom from major limb amputation and death.
Results
Downloads

Vascular Intervention
Self-expanding StentOne-handed stent release for accurate stent deployment
Vascular Intervention
Drug-Coated BalloonClinically proven to reduce restenosis and the need for reinterventions
Source:
Dr. Mwipatayi P. Presented at LINC 2015.
© BIOTRONIK AG
All rights reserved. Specifications are subject to modification, revision and improvement.
