PEACE
Pulsar efficacy - An all-comers registry: 12-month results 1
Conclusion
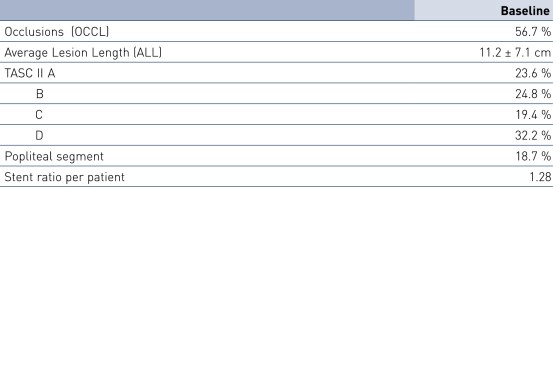
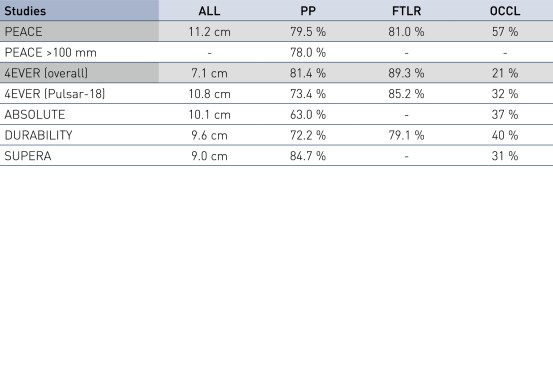
- Pulsar stents showed favorable study results at 12 months in patients with an average lesion length of 11.2 cm
- A primary patency (PP) at 12 months of 79.5% and Freedom from Target Lesion Revascularization (FTLR) of 81%
- Results are similar to published data of similar lesion lengths, including 4EVER (Pulsar-18) with a PP of 73.4% and a FTLR of 85.2%
- No significant difference between TASC A/B vs. TASC C/D lesions (p = 0.55) and diabetics vs. non-diabetics (p = 0.92)
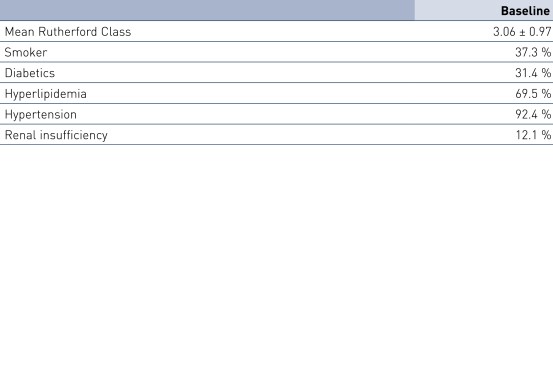
Patient Demographics
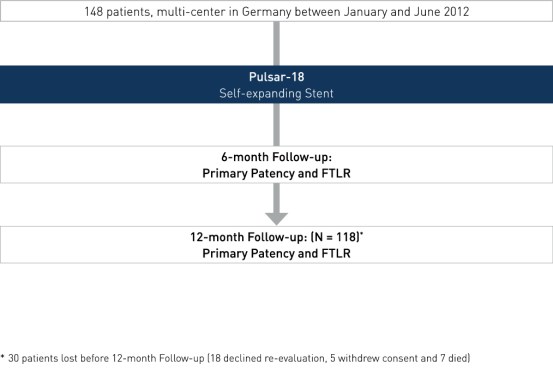
Study Design
- Multi-center, prospective all-comers registry
- Number of patients (n): 1482
- Primary investigators: Dr. Michael Lichtenberg, Arnsberg, Germany and Prof. Dr. Günther Wittenberg, Bielefeld, Germany
- Endpoints: PP3 at 6 and 12 months and FTLR
- Follow-up at 6 and 12 months
- Participating centers: Dr. B Hailer, Essen, Germany; Dr. Claus Nolte-Ernsting, Mülheim, Germany; Dr. Christiane Tiefenbacher, Wesel, Germany; Dr. Jawed Arjumand, Wuppertal, Germany; Dr. Michael Lichtenberg, Arnsberg, Germany; Dr. Günther Wittenberg, Bielefeld, Germany
Results
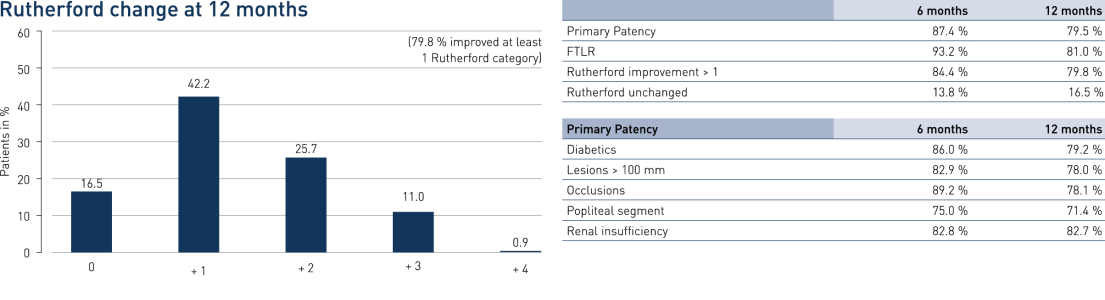
PEACE Primary Patency and Freedom from TLR in Perspective
Downloads

Vascular Intervention
Self-expanding StentOne-handed stent release for accurate stent deployment
Vascular Intervention
Self-expanding StentTri-axial shaft for a stable delivery system during stent deployment
Sources:
1 Lichtenberg M. JEVT. 2014; 21: 373-380.
2 30 patients were lost before 12-month follow-up (18 declined re-evaluation, five withdrew consent and seven died)
3 Defined as binary duplex ultrasound PSVR < 2.5 at the stented target lesion(s) and freedom from Target Lesion Revascularization (FLR)
USA: Not for sale - Investigational Device.
Limited by United States Law to Investigational Use.
© BIOTRONIK AG
All rights reserved. Specifications are subject to modification, revision and improvement.
