PEBSI RCT
NCT01839890
A Randomized Trial of Paclitaxel-Eluting Balloon after Bare Metal Stent Implantation versus Bare Metal Stent in ST Elevation Myocardial Infarction (STEMI)
Conclusion
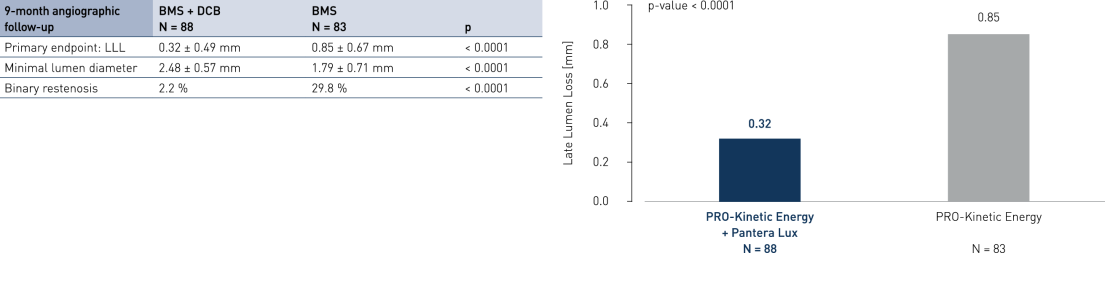
- Drug-coated balloon (DCB) after successful bare metal stent (BMS) implantation compared to BMS-only strategy in ST segment elevation myocardial infarction (STEMI) shows angiographic superiority
- Significantly inhibits neointimal growth at long follow-up
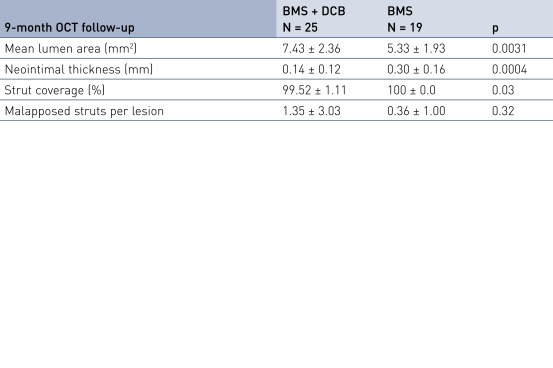
- Is associated with excellent stent strut coverage (> 99% at 9 months) and very low rates of malapposed struts
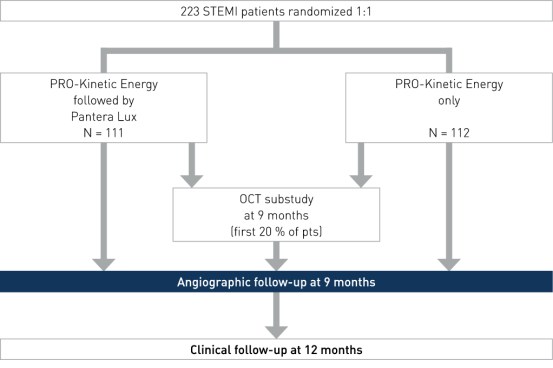
Study Design
Prospective, multi-center, randomized, investigator-initiated trial to compare the efficacy and safety of the combined treatment of BMS plus DCB versus the conventional treatment (BMS only) in patients with STEMI within 12 hours of symptom onset. Baseline characteristics were similar in both groups
- Number of patients (n): 223
- Principal investigators: Dr. Arturo Garcia-Touchard and Dr. Javier Goicolea, Hospital Universitario Puerta de Hierro, Madrid, Spain
- Primary angiographic endpoint: 9-month late lumen loss (LLL)
- Secondary imaging endpoint: 9-month OCT (substudy, first 20% of patients)
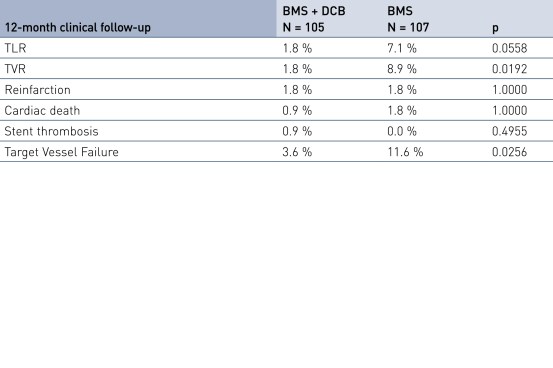
- Secondary clinical endpoints: 12-month major adverse cardiac event (MACE), target vessel failure (TVF), target vessel revascularization (TVR), target lesion revascularization (TLR), stent thrombosis (ST), stroke, bleeding
Primary Endpoint Result (9-month angiographic follow-up)
Secondary Endpoint Results
Downloads

Vascular Intervention
Cobalt Chromium Coronary Stent System60 μm thin struts for better clinically proven results
Vascular Intervention
Clinical StudyEvaluates the clinical performance of the PRO-Kinetic Energy BMS in a large real-world patient population in standard clinical care.
Sources:
Garcia-Touchard A. Presented at EuroPCR 2015.
Garcia-Touchard A. Presented at ACC 2015.
www.clinicaltrials.gov, NCT01839890
© BIOTRONIK AG All rights reserved. Specifications are subject to modification, revision and improvement.
