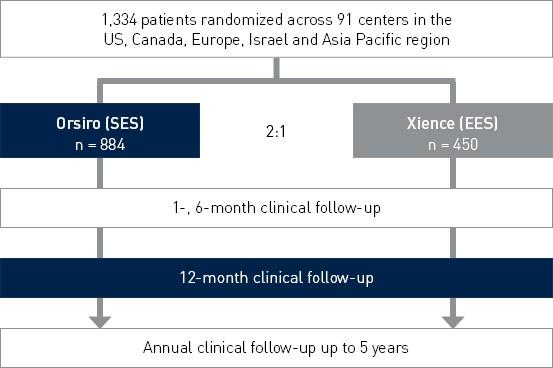
BIOFLOW-V RCT
NCT02389946
Randomized Clinical Trial Comparing Orsiro1 Drug-Eluting Stent with Xience2 DES
Conclusion
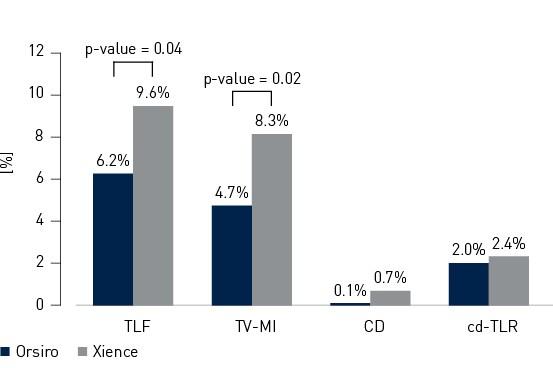
- In this 1,334 patient large, international, randomized trial Orsiro demonstrated statistically significantly lower Target Lesion Failure (TLF) rates compared to Xience at 12 months (Orsiro 6.2%, Xience 9.6%, p-value = 0.04)
- Procedure success was significantly higher with Orsiro (Orsiro 93.8%, Xience 90.1%, p-value = 0.02), mainly driven by a higher rate of in-hospital Myocardial Infarction (MI) associated with Xience (Orsiro 3.9%, Xience 6.7%, p-value = 0.03)
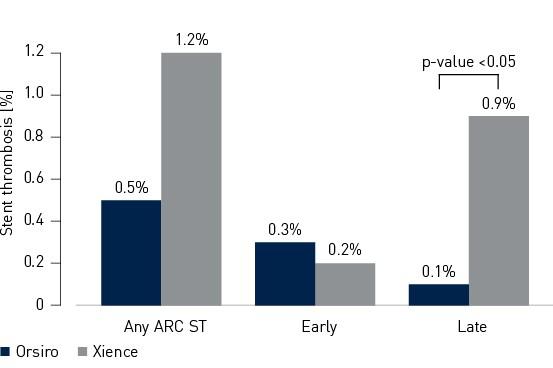
- Stent Thrombosis (ST) rate was numerically lower in the Orsiro cohort (Orsiro 0.5%, Xience 1.2%, p-value = 0.18)
Study Design
Prospective, multi-center, 2:1 randomized controlled IDE (Investigational Device Exemption) trial to assess the safety and effectiveness of Orsiro in the treatment of patients with up to three de novo or restenotic lesions (standard PTCA only).
Principal investigators:
- Dr. David Kandzari, Piedmont Heart Institute, Atlanta, US
- Dr. Jacques Koolen, Catharina Ziekenhuis, Eindhoven, Netherlands
Endpoints
Primary endpoint:
TLF at 12 months defined as a composite of Cardiac Death (CD), Target-Vessel MI (TV-MI) or any clinically-driven Target Lesion Revascularization (cd-TLR)
Pre-specified secondary endpoints:
- Components of the primary endpoint
- Target Vessel Failure (TVF) and individual TVF components
- Death
- MI and/or CD
- ST (all, definite, definite/probable, probable, possible ST)3
- Success rates (device, lesion and procedure)
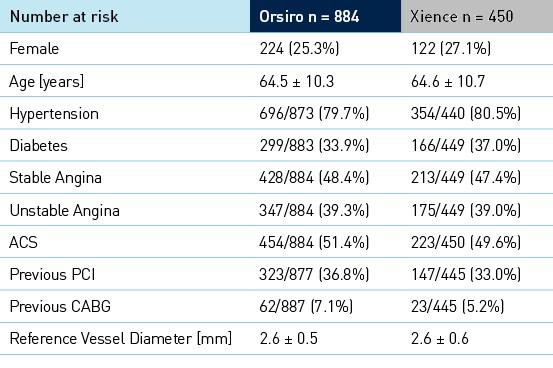
Baseline clinical, angiographic and procedural characteristics

Clinical results
Primary endpoint and composits at 12 months
Secondary endpoint – 12-month Academic Research Consortium (ARC) ST3
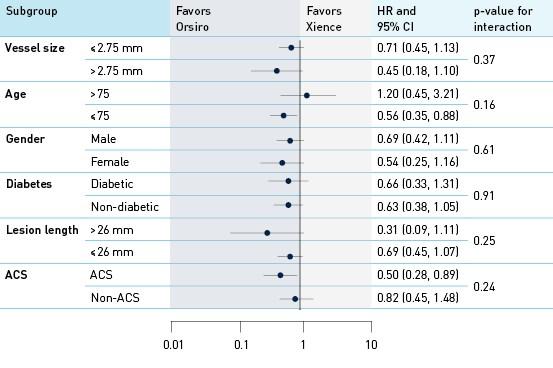
Analysis of interaction by groups and subgroups TLF at 12 months
Kaplan-Meier estimate
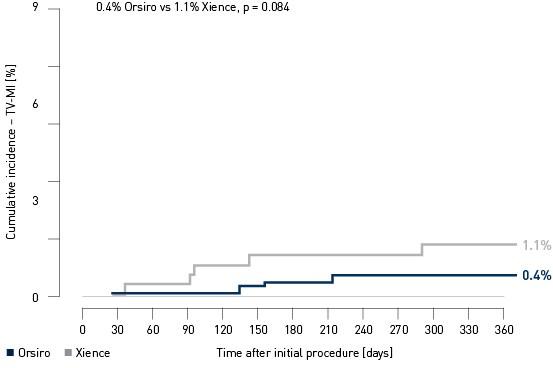
Landmark Analysis TV-MI4

Disclaimer
© BIOTRONIK AG – All rights reserved. Specifications are subject to modification, revision and improvement.
Source
Kandzari D. et al., Ultrathin Bioresorbable Polymer Sirolimus-Eluting Stents versus Thin Durable Polymer Everolimus-Eluting Stents in Patients Undergoing Coronary Revascularisation (BIOFLOW-V): a randomised trial, 2017, The Lancet.
1 CAUTION - Investigational device. Limited by United States law to investigational use.
3 according to Academic Research Consortium (ARC) criteria for acute, subacute, late, very late and cumulative stent thrombosis
2 Xience is a registered trademark of Abbott Cardiovascular Systems Inc.
4 Kandzari D. BIOFLOW-V: A Prospective Randomized Multicenter Study to Assess the SaFety and Effectiveness of the Orsiro SiroLimus Eluting Coronary Stent System in the Treatment Of Subjects With up to Three De Novo or Restenotic Coronary Artery Lesions Science. Presentation at ESC 2017.
