SORT OUT VII
NCT01879358
BIOTRONIK Sirolimus-eluting Orsiro stent vs Terumo biolimus-eluting Nobori Stent
Conclusion
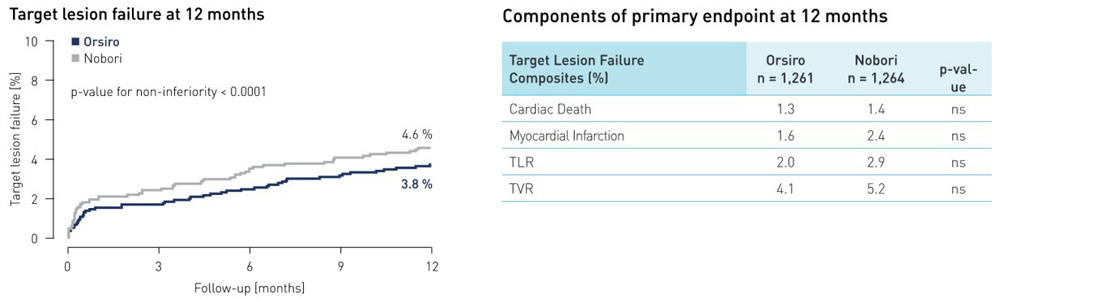
- One year data from large (n = 2,525) industry independent SORT OUT VII trial presented at EuroPCR 2015 demonstrate Orsiro is non-inferior to Nobori stent
- At one year, the primary endpoint target lesion failure (TLF) occurred in 3.8 % of Orsiro patients vs. 4.6 % of those treated with Nobori DES (p non-inferiority < 0.0001)
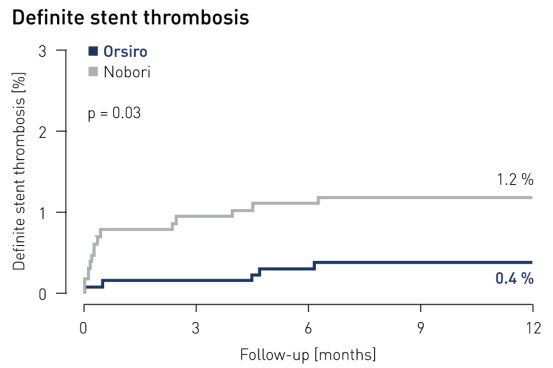
- Patients in the Orsiro arm demonstrated a significantly lower rate of definite stent thrombosis: only 0.4 % of Orsiro patients compared with 1.2 % of patients in the Nobori arm (p = 0.03)
- These highly encouraging results reconfirm those of BIOSCIENCE, the large independent comparison between Orsiro and Xience Prime presented at ESC 2014 and published in The Lancet
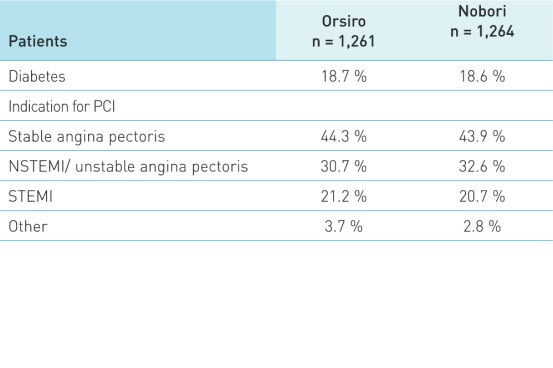
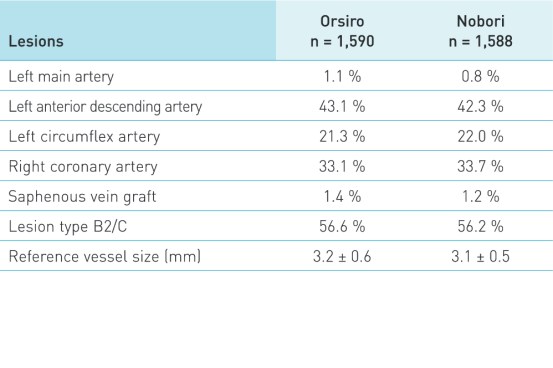
Patient and lesion characteristics
Image
Image
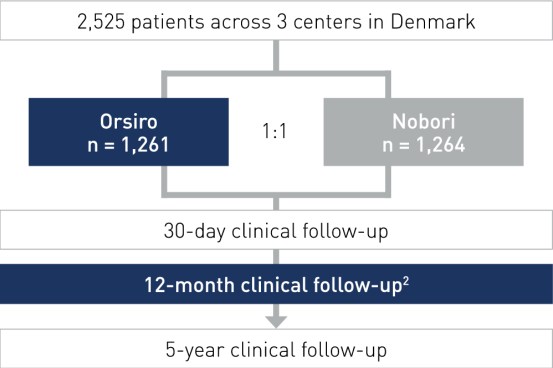
Study Design
- All-comers, multi-center, randomized, non-inferiority design
- Principal Investigators: Prof. Lisette Okkels Jensen, Odense, Denmark and Dr. Per Thayssen, Odense, Denmark
Image
Primary endpoint results
Image
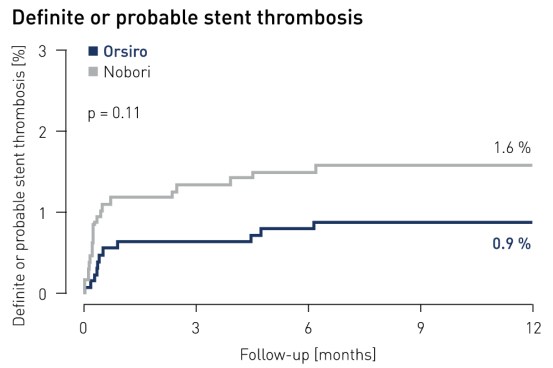
Stent thrombosis1 results at 12 months
Image
Image
Downloads

Source
Presentation, Lisette Okkels Jensen, EuroPCR 2015
Disclaimer
© BIOTRONIK AGAll rights reserved. Specifications are subject to modification, revision and improvement.
1 Definite and probable stent thrombosis according to ARC definition and adjudicated by independent clinical events committee
2 Primary endpoint: Target Lesion Failure composite of cardiac death, myocardial infarction or target lesion revascularization
