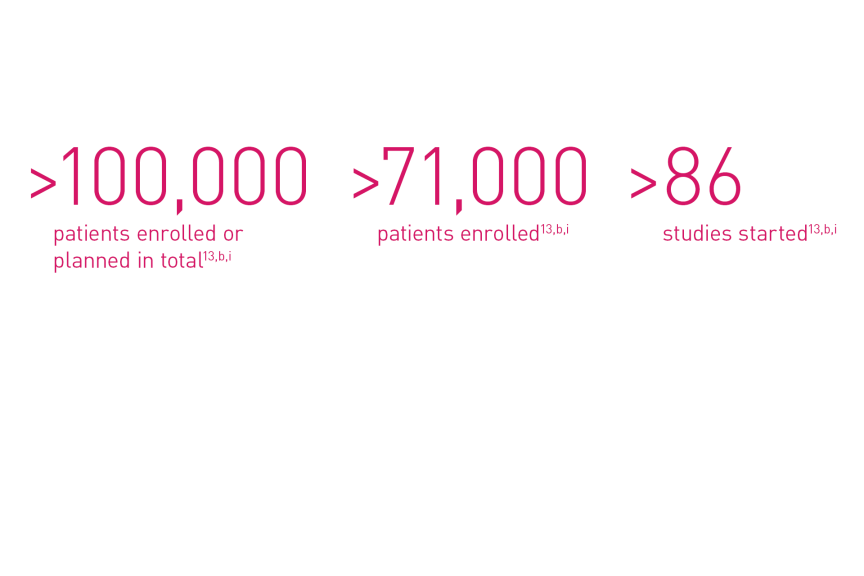Orsiro®Mission
Orsiro Mission DES is indicated for improving coronary luminal diameter in patients with symptomatic ischemic heart disease due to discrete de-novo stenotic lesions and in-stent restenotic lesions (length ≤ 40 mm) in the native coronary arteries with a reference vessel diameter of 2.25 mm to 4.0 mm including the following patient and lesion subsets:
- Acute Coronary Syndrome (ACS)
- ST-Elevation Myocardial Infarction (STEMI)
- Diabetes Mellitus (DM)
- High Bleeding Risk (HBR)
- One Month of Dual Antiplatelet Therapy (DAPT) in HBR Patients
- Calcified Lesions (moderate/severe calcification)
- Complex Lesions (B2/C)
- Long Lesions (LL) (e.g. ≥ 20 mm)
- Small Vessels (SV) (e.g. ≤ 2.75 mm)
- Multi-Vessel Disease (MVD)
- Male/Female
- Old Patients (e.g. > 65 y)
Product Highlights
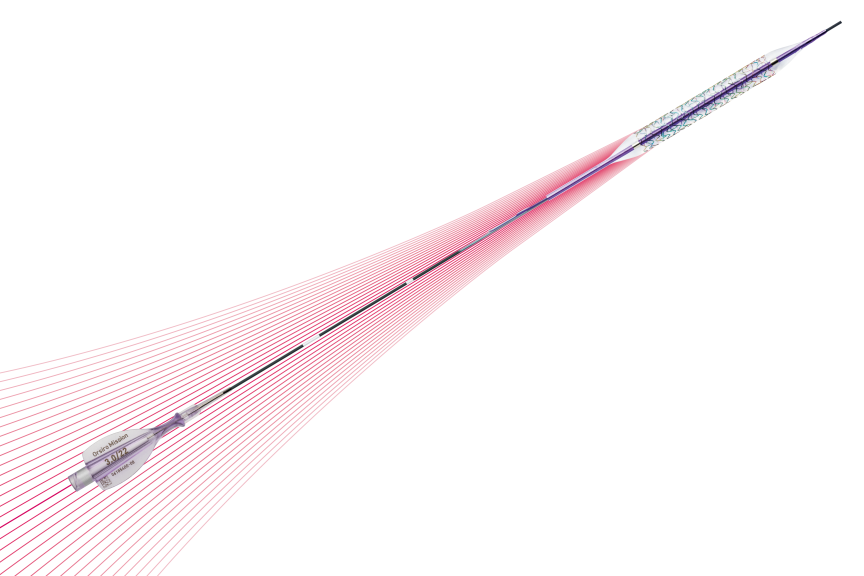
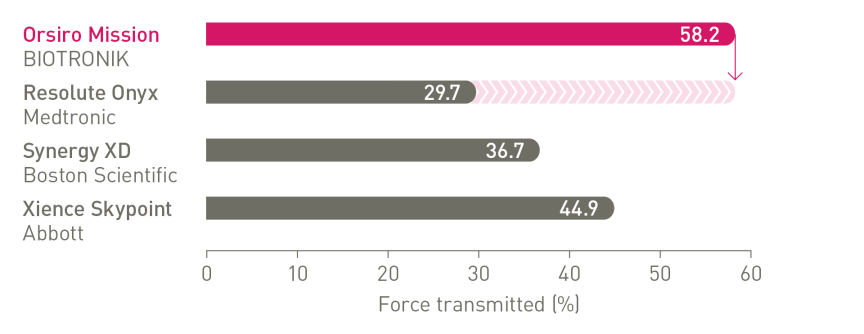
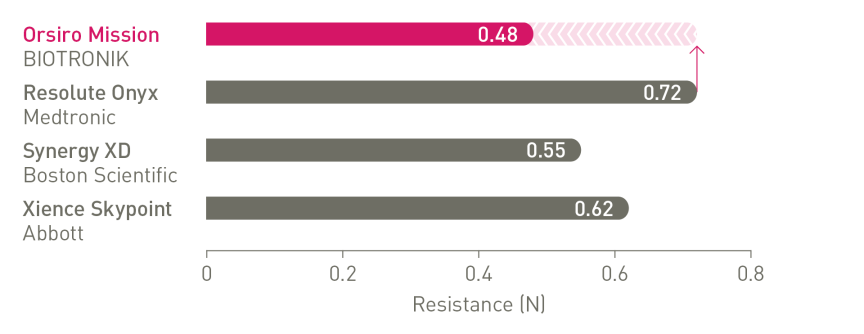
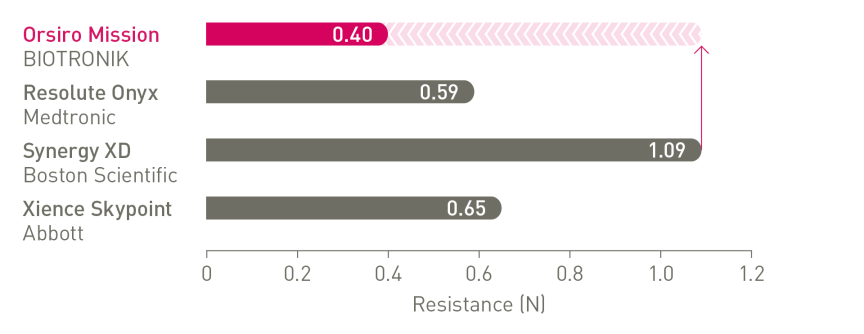
The next level of deliverability²
1st in Push3, 1st in Track3, 1st in Cross3
Ultrathin struts⁴
For early endothelialization5
Outstanding patient outcomes⁶ᵇ
Proven superiority in STEMI up to 5 years1,b,c
Orsiro family of DES – One of the most studied DES14,b,i
Continued superiority in STEMI at 5 years15,b
In the BIOSTEMI trial, Orsiro DES is proven to be safe and effective at short- and long-term follow-up with 31% significantly less Target Lesion Failure (TLF) at 5-year in STEMI patients15,b
Product Overview
Orsiro Mission
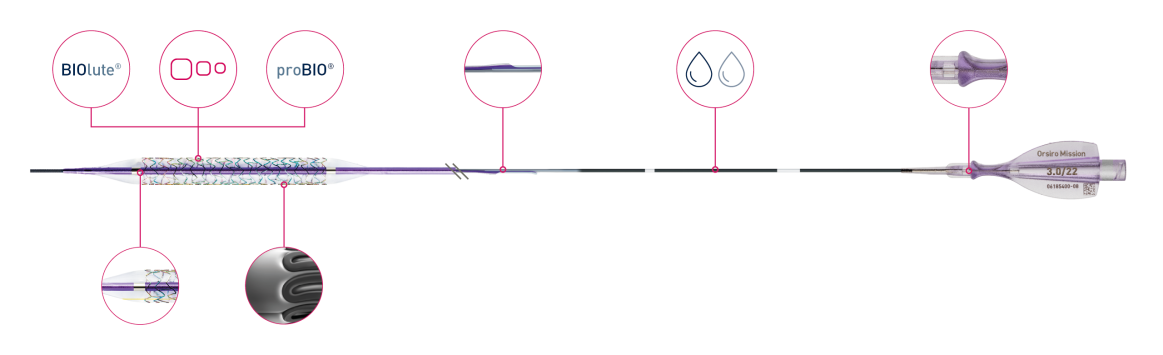
Bioabsorbable coating
Ultrathin 60 µmᶜ struts
Passive coating
Enhanced force transmission
Dual-coating
Ergonomic hub
Flexible shaft
Deep embedding
Technical Data
| Stent | |
|---|---|
| Stent material | Cobalt chromium, L-605 |
| Strut thickness | ø 2.25 – 3.0 mm: 60 µm (0.0024”); ø 3.50 – 4.0 mm: 80 µm (0.0031”) |
| Passive coating | proBIO® (Amorphous Silicon Carbide) |
| Active coating | BIOlute® bioabsorbable Poly-L-Lactide (PLLA) eluting a limus drug |
| Drug dose | 1.4 µg/mm² |
| Delivery system | |
| Catheter type | Rapid exchange |
| Recommended guide catheter | 5F (min. I.D. 0.056”) |
| Guide wire diameter | 0.014” |
| Usable catheter length | 140 cm |
| Balloon material | Semi crystalline polymer material |
| Coating (Distal shaft) | Hydrophilic |
| Coating (Proximal shaft) | Hydrophobic |
| Marker bands | Two swaged platinum-iridium markers |
| Lesion entry profile | 0.017” |
| Distal shaft diameter | 2.7F: ø 2.25 – 3.0 mm; 2.9F: ø 3.5 – 4.0 mm |
| Proximal shaft diameter | 2.0F |
| Nominal pressure (NP) | 10 atm |
| Rated burst pressure (RBP) | 16 atm |
| Storage | |
|---|---|
| Use Before Date (UBD) | 24 months |
| Temperature | Between 15°C (59°F) and 25°C (77°F), short term excursions between 10°C (50°F) and 40°C (104°F) are allowed |
| Double Helix Stent Design | ||||||
|---|---|---|---|---|---|---|
| Nominal diameter (mm) | 2.25 | 2.5 | 2.75 | 3.0 | 3.5 | 4.0 |
| Strut thickness (μm) | 60 | = | = | = | 80 | = |
| Strut width (μm) | 75 | = | = | = | 85 | = |
| Amount of connectors | 3 | = | = | = | = | = |
| Amount of crowns at end | 8 | = | = | = | = | = |
| Maximal Expansion and Stent Strut Opening | ||||||
|---|---|---|---|---|---|---|
| Nominal diameter (mm) | 2.25 | 2.5 | 2.75 | 3.0 | 3.5 | 4.0 |
| Nominal outer diameter of the stent at NP (mm) | 2.37 | 2.62 | 2.87 | 3.12 | 3.66 | 4.16 |
| Maximal expansion diameter (mm) | 4.0 | = | = | = | 5.0 | = |
| Stent strut opening diameter at NP* (mm) | 0.79 | 0.92 | = | = | 1.06 | 1.25 |
| Maximal diameter of expanded stent cell (mm) | 3.59 | = | = | = | 4.42 | = |
*Mean of the largest possible opening diameter within a stent cell at NP
= symbol used to show repetition
Ordering Information
| Stent ø (mm) |
Stent Length (mm) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 9 | 13 | 15 | 18 | 22 | 26 | 30 | 35 | 40 | |
| 2.25 | 419101 | 419107 | 419113 | 419119 | 419125 | 419131 | 419137 | 419143 | 419149 |
| 2.5 | 419102 | 419108 | 419114 | 419120 | 419126 | 419132 | 419138 | 419144 | 419150 |
| 2.75 | 419103 | 419109 | 419115 | 419121 | 419127 | 419133 | 419139 | 419145 | 419151 |
| 3.0 | 419104 | 419110 | 419116 | 419122 | 419128 | 419134 | 419140 | 419146 | 419152 |
| 3.5 | 419105 | 419111 | 419117 | 419123 | 419129 | 419135 | 419141 | 419147 | 419153 |
| 4.0 | 419106 | 419112 | 419118 | 419124 | 419130 | 419136 | 419142 | 419148 | 419154 |
Downloads and Related Links
Downloads
Related Links
Media
Product Animations
How can we help you?
References
a. BIOTRONIK data on file (n = 5), based on statistically significant differences on the bench for Pushability, Trackability, and Crossability compared to Xience Skypoint, superior to Xience in STEMI patients; b. Clinical data collected with Orsiro DES device within the Orsiro DES family clinical program; c. In comparison to Xience, based on TLF with Orsiro DES; d. In comparison to Resolute Onyx, BIOTRONIK data on file; e. In comparison to Synergy XD, BIOTRONIK data on file; f. Always refer to the Instructions for Use (IFU) for the Maximum Diameter for post-dilation applying in your country; g. Ø 2.25-3.0 mm strut thickness 60 µm, Ø 3.5-4.0 mm strut thickness 80 µm; h. Images: Secco G et al. Time-related changes in neointimal tissue coverage following a new generation SES implantation: an OCT observational study. Presented at: euro PCR, May 20, 2014; Paris, France, i. Clinical data collected with Orsiro Mission DES device within the Orsiro DES family clinical program; j. At 5-year in STEMI patients.
1. Iglesias JF. et al, Long-term outcomes with biodegradable polymer sirolimus eluting stents versus durable polymer everolimus-eluting stents in ST-segment elevation myocardial infarction: 5-year follow-up of the BIOSTEMI randomized superiority trial, The Lancet, 2024; 2. In comparison to Xience Sierra, Resolute Onyx and Synergy for bench tests on pushability, trackability and crossability, BIOTRONIK data on file; 3. In comparison to Resolute Onyx, Xience Sierra and Synergy, BIOTRONIK data on file; 4. As characterized with respect to strut thickness in Bangalore et al. Meta-analysis; 5. Per investigators’ interpretation in Secco et al. Imaging data serial observations. Secco GG et al. Time-related changes in neointimal tissue coverage of a novel Sirolimus eluting stent: Serial observations with optical coherence tomography. Cardiovascular Revascularization Medicine. 2016; 17(1): 38-43; 6. Based on investigator’s interpretation of BIOFLOW-V endpoint results; 7. Based on Kapoor A. et al., The road to the ideal stent: A review of stent design optimization methods, findings, and opportunities, Materials&Design, 2024; 8. Stefanini GG et al. Coronary stents: novel developments. Heart. 2014 Jul 1;100(13):1051-61; 9. Low AF. Stent platform for procedural success: Introducing the Continuous Sinusoidal & Core Wire Technologies. Presented at: AsiaPCR; 22-24 January, 2015; Singapore, Singapore; 10. Tolentino A. Evolving DES Strategy: Biodegradable Polymer vs. Bioabsorbable Scaffold. Presented at: Cardiovascular Nurse/Technologist Symposium; June 17, 2016; New York, USA; 11. Secco G et al. Time-related changes in neointimal tissue coverage of a novel Sirolimus eluting stent: Serial observations with optical coherence tomography. Cardiovascular Revascularization Medicine 17.1 (2016): 38-43; 12. Pilgrim et al. Biodegradable – versus durable-polymer drug-eluting stents for STEMI. Final 2-year outcomes of the BIOSTEMI trial. J Am Coll Cardiol. Cardiovasc Interven. 2021, doi: 10.1016/j.jcin.2020.12.011; 13. BIOTRONIK data on file, status February 2023; 14. In large RCTs, based on Taglieri et al. Meta-analysis, against currently used DES; 15. Based on TLF primary endpoint. Iglesias, JF. et al. Long-term outcomes with biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stents in ST-segment elevation myocardial infarction: 5-year follow-up of the BIOSTEMI randomized superiority trial, presented at TCT 2023; 16. Kandzari D et al. Ultrathin Bioresorbable Polymer Sirolimus-Eluting Stents versus Thin Durable Polymer Everolimus-Eluting Stents for Coronary Revascularization: Final 5-year Outcomes from the Randomized BIOFLOW V Trial, Submitted manuscript to JACC, 2022: NCT02389946; 17. Valgimigli M et al. Circulation 2023 Aug 25, 18. Taglieri N et al. Target lesion failure with current drug-eluting stents evidence from a comprehensive network meta-analysis. JACC 2020.
Orsiro, Orsiro Mission, proBIO and BIOlute are trademarks or registered trademarks of the BIOTRONIK Group of Companies. All other trademarks are the property of their respective owners.