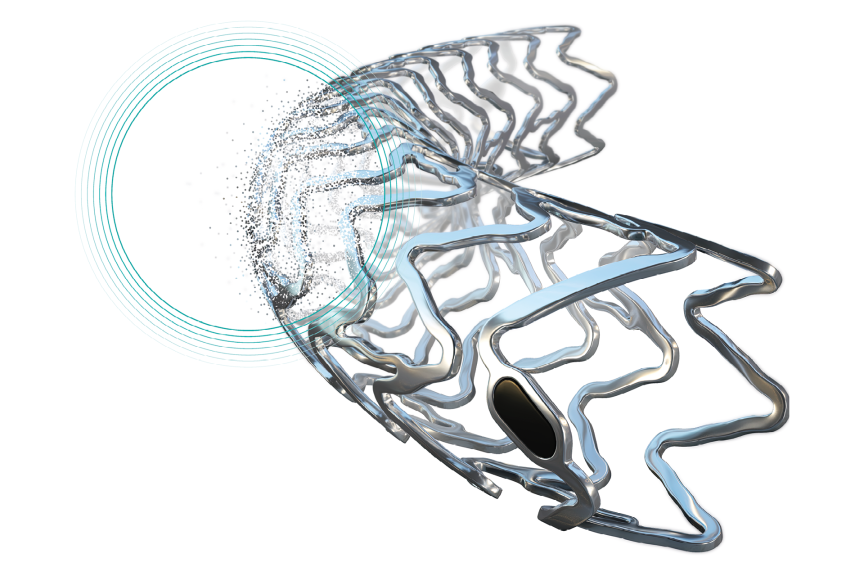
BIOTRONIK Introduces Two New Indications for the Ultrathin Strut Orsiro® Mission Drug-Eluting Stent CE-approved indications provide shortened dual antiplatelet therapy for high bleeding risk patients and treatment for moderate to severe calcified lesions
BIOTRONIK, a global leader in cardiovascular, endovascular, and neuromodulation solutions, is pleased to announce today the approval of two new indications for the Orsiro® Mission Drug-Eluting Stent (DES):
- One-month Dual Antiplatelet Therapy (DAPT) for High Bleeding Risk (HBR) Patients: Allows practitioners to offer personalized, shorter DAPT durations to their patients at high risk of bleeding events, in accordance with the most recent guidelines.
- Calcified Lesions Treatment: Allows practitioners to target more complex lesions with moderate or severe calcification.
These new indications complement the recent global regulatory approval of the Orsiro® Mission DES for an increased maximum allowed diameter (MAD) post-dilation (Ø2.25-3.0 mm, MAD: 4.0 mm, Ø3.5-4.0 mm, MAD: 5.0 mm).
Following these approvals, labelling including adapted Instructions for Use will become effective in the coming months, pending further national regulatory approvals.
Please refer to the Instructions for Use for indications and post-procedure antiplatelet therapy recommendations applicable in the respective country.
-END-
Disclaimer: Orsiro and Orsiro Mission are trademarks or registered trademarks of the BIOTRONIK Group of Companies.
More information on Orsiro® Mission DES here.
About BIOTRONIK
At BIOTRONIK, patient well-being is our top priority and has been for over 60 years. BIOTRONIK is a leading global medical technology company with products and offerings that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation therapies. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries across the Americas, EMEA (Europe, the Middle East, and Africa), and Asia-Pacific.
Related Press Releases
-
Image
 BUELACH, SwitzerlandPress Release
BUELACH, SwitzerlandPress ReleaseBIOTRONIK Begins BIOMAG-LL Pre-Market Trial to Evaluate Freesolve® Resorbable Magnesium Scaffold for Treatment of Long Lesions
BIOTRONIK, a global leader in cardiovascular, endovascular, and neuromodulation solutions, today announces the launch of BIOMAG-LL, a pre-market trial focused on confirming the safety and clinical performance of Freesolve ® Resorbable Magnesium Scaffold (RMS), for the treatment of long de novo lesions in native coronary arteries. The BIOMAG-LL is a prospective, international, multi-center, single arm pre-market study that will enroll 100 patients in Europe with de novo coronary artery stenosis and long lesions. Given that the Freesolve RMS is already CE-certified for shorter lesions, the
-
ImageBUELACH, SwitzerlandPress Release
BIOTRONIK Launches FlowGuide and Guidion Short, the Next Generation of Guide Extension Catheters
BIOTRONIK today announces the launch of the latest innovations in guide extension catheters: FlowGuide and Guidion Short. These new devices have been developed to offer enhanced support and to facilitate the delivery of devices during complex vascular interventions. The catheters introduce key elements, such as perfusion holes in the distal shaft of the FlowGuide catheter, and a transradial access and trapping friendly design for both new products. Through our collaboration with IMDS, the FlowGuide and Guidion Short catheters expand BIOTRONIK's portfolio of high-performance devices dedicated
-
Image
 BUELACH, SWITZERLANDPress Release
BUELACH, SWITZERLANDPress ReleaseBIOTRONIK Introduces Expanded Maximum Diameter Dimensions for Orsiro Mission DES
BIOTRONIK is pleased to announce the availability of an expanded Maximum Allowed Diameters (MAD) range for the Orsiro® Mission Drug Eluting Stent (DES). Diameters 2.25mm to 3.00mm of the Orsiro Mission DES can now be extended up to 4.0mm, while diameters 3.5mm and 4.0mm can reach up to 5.0mm. This new MAD expansion follows the approvals from CE, FDA, and Health Canada obtained in the past months. This update reflects a global effort for the Orsiro Mission stent technology to match contemporary Percutaneous Coronary Intervention (PCI), enabling practitioners to optimize vessel apposition and
-
Image
 BUELACH, SWITZERLANDPress Release
BUELACH, SWITZERLANDPress ReleaseOne-Year BIONETIC-I Study Results Show Safety and Effectiveness of Iliac Artery Treatment With BIOTRONIK’s Dynetic-35 Cobalt Chromium Balloon-Expandable Stent System
BIOTRONIK announced the presentation of the 12-month results from the BIONETIC-I study this week at LINC 2024. The prospective, international, multicenter single-arm observational study evaluated the treatment of de novo, restenotic or occluded iliac lesions in 160 patients with Rutherford Class 2-6 peripheral artery disease using the Dynetic ®-35 cobalt chromium balloon-expandable stent system. At baseline, 12.5% of enrolled patients had critical limb ischemia, 90% had calcified lesions (30.7% severe calcification), and there was an average of 85.5% stenosis in the target lesion. The primary
-
Image
 BUELACH, SWITZERLANDPress Release
BUELACH, SWITZERLANDPress ReleaseNew-Gen Resorbable Magnesium Scaffold Freesolve Enters BIOMAG-II Randomized Controlled Trial (RCT)
BIOTRONIK announced today the enrollment of the first patient in the BIOMAG-II trial aiming to evaluate the safety and clinical performance of its new-generation resorbable magnesium scaffold (RMS), Freesolve™, compared to a contemporary drug-eluting stent (DES). "We are delighted to enroll the first patient in the BIOMAG-II trial, which may play a critical role in helping establish resorbable metallic scaffolds as part of clinical practice in the future and I am particularly encouraged by the implantation results of the Freesolve resorbable magnesium scaffold," said Dr. Juan F. Iglesias, HUG
-
Image
 BÜLACH, SwitzerlandPress Release
BÜLACH, SwitzerlandPress ReleaseFDA Breakthrough Device Designation for BIOTRONIK Freesolve™ Below-the-Knee Resorbable Magnesium Scaffold (RMS)
BIOTRONIK has been granted Breakthrough Device Designation (BDD) from the US Food and Drug Administration (FDA) for the Freesolve™ below-the-knee resorbable magnesium scaffold (BTK RMS). The Freesolve BTK RMS is designed for individuals suffering from chronic limb-threatening ischemia (CLTI). CLTI is the most severe form of peripheral arterial disease (PAD), estimated to affect 11% of the 200 million people suffering from PAD globally. CLTI is associated with high rates of amputation and mortality as well as high care costs. 1 To qualify for a Breakthrough Device Designation, a device
-
Image
 BÜLACH, SwitzerlandPress Release
BÜLACH, SwitzerlandPress ReleaseCE-approval for BIOTRONIK’s Next-Gen Metallic Bioresorbable Scaffold Freesolve
BIOTRONIK announces the CE approval and launch of Freesolve™ Resorbable Magnesium Scaffold (RMS). This third generation RMS has been engineered to provide optimized vessel support, yet achieves magnesium resorption within 12 months. 1 The new Freesolve RMS is a groundbreaking vascular advancement based on reliable clinical evidence. Recent BIOMAG-I trial data highlights an exceptional 99.3% magnesium strut degradation 12 months after implantation 2, consistent performance, regardless of lesion characteristics, and restoration of vasomotion. 3 "Having closely observed the evolution of
-
Image
 BÜLACH, SwitzerlandPress Release
BÜLACH, SwitzerlandPress ReleaseBIOTRONIK and IMDS Join Forces to Launch Innovative Micro Rx Catheter
BIOTRONIK is proud to introduce the Micro Rx™ catheter, a novel rapid exchange microcatheter developed to easily enhance guidewire support during percutaneous coronary interventions (PCI). This cutting-edge device, exclusively distributed by BIOTRONIK, is manufactured by IMDS (Interventional Medical Device Solutions). Micro Rx catheter marks the fourth IMDS product BIOTRONIK has brought to the U.S., creating a compelling portfolio of devices which includes NHancer Rx, TrapIT, and ReCross catheters. The Micro Rx catheter features a reinforced distal shaft with a core wire between two layers of
-
Image
 BÜLACH, SwitzerlandPress Release
BÜLACH, SwitzerlandPress ReleaseTwo-Year BIOPACT Randomized Controlled Trial (RCT) Analysis Demonstrates “Persistent Excellence” for Low-Profile Passeo-18 Lux DCB
BIOTRONIK announced the presentation of two-year results from the investigator-initiated BIOPACT RCT by Principal Investigator Dr. Koen Deloose at the Paris Vascular Insights 2023 congress. The randomized controlled non-inferiority trial evaluated the safety and efficacy of the Passeo®-18 Lux® drug-coated balloon (DCB) catheter compared to the In.Pact Admiral DCB (Medtronic), and showed excellent results for both balloons through 24 months. 1 The prospective, multicenter, core-lab adjudicated non-inferiority trial enrolled 302 patients in Austria, Belgium, France and Switzerland with
-
Image
 COPENHAGEN, DenmarkPress Release
COPENHAGEN, DenmarkPress ReleaseBIOLUX P-III BENELUX All-Comers Registry Demonstrates Safety and Efficacy of BIOTRONIK’s Drug-Coated Balloon in Isolated Popliteal Lesions at 24 Months
BIOTRONIK announced the two-year-results from the BIOLUX P-III BENELUX all-comers registry, presented by Principal Investigator Prof. Frank Vermassen at CIRSE 2023 in Copenhagen, Denmark. 1 The prospective, international, multicenter post-market registry evaluated the safety and efficacy of the Passeo ®-18 Lux ® drug-coated balloon (DCB) catheter in isolated popliteal artery lesions. This indication is considered a difficult vessel bed to treat due to its biomechanical constraints that usually preclude the placement of stents. BIOLUX P-III BENELUX registry enrolled 99 patients in Belgium, the
-
Image
 AMSTERDAM, The NetherlandsPress Release
AMSTERDAM, The NetherlandsPress ReleaseBIOMAG-I Study OCT Analysis Confirmed That BIOTRONIK’s Newest Resorbable Magnesium Scaffold Resorbed at 12 Months
New data from the BIOMAG-I first-in-human trial shed light on the vascular healing process following the implantation of DREAMS 3G, BIOTRONIK’s third-generation resorbable magnesium scaffold (RMS). A detailed intravascular optical coherence tomography (OCT) analysis demonstrated that 99.3% of the struts completely degraded at one year. 1 Dr. Masaru Seguchi from the German Heart Centre in Munich, Germany presented the findings at the European Society of Cardiology’s (ESC) congress in Amsterdam. While earlier BIOMAG-I study results provided favorable outcomes with regards to late lumen loss at
-
Image
 AMSTERDAM, The NetherlandsPress Release
AMSTERDAM, The NetherlandsPress Release12-Month-Data of BIOFLOW-DAPT Study Show Positive Results for Orsiro Mission DES With Short DAPT
BIOFLOW-DAPT one-year-data demonstrated non-inferiority and a good safety profile for the Orsiro ® Mission drug-eluting stent (DES) compared to Resolute Onyx DES (p<0.0001) in patients at high risk of bleeding receiving short dual antiplatelet therapy (DAPT). Prof. Marco Valgimigli presented the novel data in a late-breaking trial session at the European Society of Cardiology’s (ESC) Congress in Amsterdam. The results were published simultaneously in Circulation. BIOFLOW-DAPT is a prospective, multi-center, international, two-arm randomized controlled clinical study to assess the safety of one
-
Image
 BUELACH, SwitzerlandPress Release
BUELACH, SwitzerlandPress ReleaseBIOPACT RCT Subgroup Analyses Show Consistently Great Results for Passeo-18 Lux Drug-Coated Balloon
BIOTRONIK announced the one-year subgroup results from the investigator-initiated BIOPACT randomized controlled trial (RCT), which were presented by principal investigator Dr. Koen Deloose at LINC, the Leipzig Interventional Course 2023. The randomized controlled non-inferiority trial evaluated the safety and efficacy of the Passeo ®-18 Lux ® drug-coated balloon (DCB) catheter compared to the In.Pact Admiral DCB (Medtronic) and showed excellent results for both balloons through 12 months across a variety of sub-cohorts. The prospective, multicenter, core-lab adjudicated non-inferiority study
-
Image
 BUELACH, SwitzerlandPress Release
BUELACH, SwitzerlandPress ReleaseLate-Breaking Study Data: BIOTRONIK’s Orsiro DES Outperforms Other Ultrathin Strut Drug-Eluting Stent
In a late breaking trial session during EuroPCR 2023 in Paris, on behalf of the HOST-IDEA study investigators, Dr. Hyo-Soo Kim presented the results of a stent level analysis comparing two ultrathin strut drug-eluting stents (DES): Orsiro ® and Coroflex ISAR. The post-hoc comparison revealed significant differences in efficacy. HOST-IDEA is a large scale, multicenter, all-comers randomized controlled trial that demonstrated the non-inferiority of 3- to 6-month versus 12-month dual antiplatelet therapy (DAPT) after implantation of ultrathin strut DES. 2,173 patients in 37 South Korean centers
-
Image
 BUELACH, SwitzerlandPress Release
BUELACH, SwitzerlandPress ReleaseBIOTRONIK Launches Oscar Multifunctional Peripheral Catheter at LINC 2023
BIOTRONIK is pleased to announce the limited release of its Oscar ® ( One Solution: Cross. Adjust. Restore) multifunctional peripheral catheter and start of promotional activities at LINC, the Leipzig Interventional Course held June 6-9 in Leipzig, Germany. As indicated per Instructions for Use, the Oscar catheter is intended for dilation of stenotic segments in peripheral vessels. The device is comprised of three fully user-adjustable components (support catheter with integrated Lock Grip, extendable dilator and length-adjustable PTA balloon). It was developed to provide support during access
-
Image
 PARIS, FrancePress Release
PARIS, FrancePress ReleaseBIOMAG-I 12-Month Study Data Highlights Continued Excellent Patient Outcomes With New DREAMS 3G Scaffold
In the first-in-human study BIOMAG-I, BIOTRONIK’s new-generation DREAMS 3G resorbable magnesium scaffold (RMS) showed significantly lower in-scaffold late lumen loss (LLL) than its predecessor at 12 months as well as excellent safety and efficacy. Prof. Michael Haude, BIOMAG-I Coordinating Clinical Investigator, presented the latest results in the late breaking trial session at the EuroPCR course. 1 At one-year follow-up, BIOMAG-I data confirmed the excellent safety profile of DREAMS 3G RMS with a low target lesion failure rate of 2.6%. Neither cardiac death and myocardial infarction occurred
-
Image
 BUELACH, SwitzerlandPress Release
BUELACH, SwitzerlandPress ReleaseNew Data Highlight Promising Angiographic and Safety Profile of BIOTRONIK’s Third Generation Resorbable Magnesium Scaffold
Prof. Michael Haude, BIOMAG-I Coordinating Clinical Investigator, presented the latest results of the BIOMAG-I clinical study at the Cardiovascular Research Technologies (CRT) meeting. 1 At six months the angiographic and clinical data showed a low in-scaffold late lumen loss (LLL) rate and a good safety profile with no scaffold thrombosis. A low proportion of mal-apposed struts after implantation was observed, at six months struts were no longer discernable. The intravascular imaging documented a preservation of the scaffold area with a low mean neointimal area. Bioresorbable scaffolds have
-
Image
 BUELACH, SwitzerlandPress Release
BUELACH, SwitzerlandPress ReleaseBIOTRONIK Announces Its Latest Innovation: Oscar Multifunctional Peripheral Catheter
BIOTRONIK announces the FDA 510(k) clearance and CE mark of its Oscar ® ( One Solution: Cross. Adjust. Restore) multifunctional peripheral catheter. Physicians in the U.S. have already used the novel device in more than 70 cases. As indicated per Instructions for Use, Oscar is intended for percutaneous transluminal interventions in the peripheral vasculature. The device was developed to provide support during access into and to dilate stenoses in femoral, popliteal and infrapopliteal arteries. The Oscar peripheral multifunctional catheter is comprised of three user-adjustable components: The
