BIOTRONIK and Texray Establish Collaboration to Distribute Texray’s Innovative Radiation Protection Products New Partnership Makes Outstanding Radiation Protection Solutions Available to Healthcare Professionals
BIOTRONIK, a leading global medical technology company specializing in innovative cardiovascular and endovascular solutions, proudly announces a multinational distribution partnership with Texray, the pioneering developer of a groundbreaking radiation protection textile. In this collaboration BIOTRONIK will distribute Texray's head and neck protectors starting in selected European and Middle Eastern countries. Both partners aim to raise awareness about the critical importance of radiation protection for healthcare professionals, who serve in environments where radiation exposure is an inherent occupational risk.
“Texray's radiation protective textiles complement BIOTRONIK’s product portfolio, enhancing our ability to offer customers comprehensive protection solutions. We are delighted to expand our offering, ensuring that healthcare professionals have a range of radiation protection options to support their safety in their daily work,” says John Brumfield, Global Vice President Marketing CRM at BIOTRONIK.
Proprietary High-Quality Material
Texray's protective products feature a patented technological textile platform. This technology allows for designs that fulfill the demand for more functional and comfortable radiation protection products. The garments enhance ergonomic parameters, and exhibit improved durability compared to the traditional "rubber-sheet" garments.
Innovative Swedish Design
Texray offers two types of radiation protective products: HeadPeace, a comfortable headband to reduce radiation in the upper section of the head, and MindPeace, a uniquely designed thyroid collar with an extended panel to reduce radiation in the lower and middle section of the head1. Combined, these products reduce radiation dose to the neck and head by up to 97% in compared to using only a standard thyroid collar2.
"BIOTRONIK, with its legacy in providing radiation protection solutions since 2011, has recognized the value of Texray's innovations. Our cutting-edge innovations will now be accessible for healthcare professionals through the experienced BIOTRONIK clinical and sales forces around the globe. We are excited about having this opportunity with a partner such as BIOTRONIK, that shares our vision of improving occupational safety for the ones who save the lives of others," says Mrs. Petra Apell, Founder and CEO of Texray.
-END-
References:
1. Larsson, M.E., Jonasson, P.I., Apell, P.S. et al. Evaluation of novel radiation protection devices during radiologically guided interventions. CVIR Endovasc 7, 18 (2024).
2. Bärenfänger, F., Walbersloh, J., El Mouden R., Goerg, F., Block, A., Rohde, S., Clinical evaluation of a novel head protection system for interventional radiologists. European Journal of Radiology 2022:147.
About BIOTRONIK:
At BIOTRONIK, patient well-being is our top priority and has been for 60 years. BIOTRONIK is a leading global medical technology company with products and services that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation solutions. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries.
More Press Releases
-
ImageLAKE OSWEGO, Oregon, USAPress Release
BIOTRONIK Neuro Launches BioVantage™ Remote Care Review
BIOTRONIK Neuro today announced an entirely new service option for Prospera™ patients, called BioVantage™. This expanded service option complements Prospera's daily remote monitoring by providing patients a remote, monthly check-in with an experienced spinal cord stimulation (SCS) specialist from BIOTRONIK Neuro’s Embrace One™ Care Team. The goal is to enhance patient therapy education and support, promoting not just optimal pain relief but also consistent progress toward the treatment plan. Ultimately, BioVantage aims to prolong patient engagement and compliance. Remote patient management has
-
ImageBUELACH, SwitzerlandPress Release
BIOTRONIK Launches FlowGuide and Guidion Short, the Next Generation of Guide Extension Catheters
BIOTRONIK today announces the launch of the latest innovations in guide extension catheters: FlowGuide and Guidion Short. These new devices have been developed to offer enhanced support and to facilitate the delivery of devices during complex vascular interventions. The catheters introduce key elements, such as perfusion holes in the distal shaft of the FlowGuide catheter, and a transradial access and trapping friendly design for both new products. Through our collaboration with IMDS, the FlowGuide and Guidion Short catheters expand BIOTRONIK's portfolio of high-performance devices dedicated
-
Image
 MONTREAL, CanadaPress Release
MONTREAL, CanadaPress ReleaseAmvia Sky Launched in Canada, the World’s First Pacemaker Approved for LBBAP*
Dr. Fadi Mansour performed the first Canadian implant of BIOTRONIK’s newest pacemaker and CRT-P generation earlier this year at the Centre Hospitalier de l'Université de Montréal. The patient received an Amvia Sky HF-T QP triple chamber pacemaker device. The first implant follows the full market release of BIOTRONIK’s newest family of pacemaker and CRT-P devices, featuring patient-centric technologies for better patient care and simplified workflows. “The Amvia Sky HF-T QP offers a significant number of advanced features, making it the most complete CRT-P on the market including streamlined
-
Image
 BUELACH, SWITZERLANDPress Release
BUELACH, SWITZERLANDPress ReleaseOne-Year BIONETIC-I Study Results Show Safety and Effectiveness of Iliac Artery Treatment With BIOTRONIK’s Dynetic-35 Cobalt Chromium Balloon-Expandable Stent System
BIOTRONIK announced the presentation of the 12-month results from the BIONETIC-I study this week at LINC 2024. The prospective, international, multicenter single-arm observational study evaluated the treatment of de novo, restenotic or occluded iliac lesions in 160 patients with Rutherford Class 2-6 peripheral artery disease using the Dynetic ®-35 cobalt chromium balloon-expandable stent system. At baseline, 12.5% of enrolled patients had critical limb ischemia, 90% had calcified lesions (30.7% severe calcification), and there was an average of 85.5% stenosis in the target lesion. The primary
-
Image
 BUELACH, SWITZERLANDPress Release
BUELACH, SWITZERLANDPress ReleaseBIOMAG-I Two-Year Study Results Confirm Excellent Safety and Efficacy Profile for Freesolve, BIOTRONIK’s Newest Resorbable Magnesium Scaffold Innovation
New two-year follow-up data from the BIOMAG-I first-in-human trial confirms a reliable and predictable long-term safety profile for Freesolve™, the third-generation resorbable magnesium scaffold (RMS), establishing it as a true alternative to contemporary drug-eluting stents (DES). The results were presented by Prof. Dr. Michael Haude at the EuroPCR 2024 conference yesterday in Paris. At the 24-month follow-up, the incidence of target lesion failure (TLF) was 3.5% alongside a corresponding 3.5% incidence of target lesion revascularization (TLR) compares favorably with various second-generation
-
Image
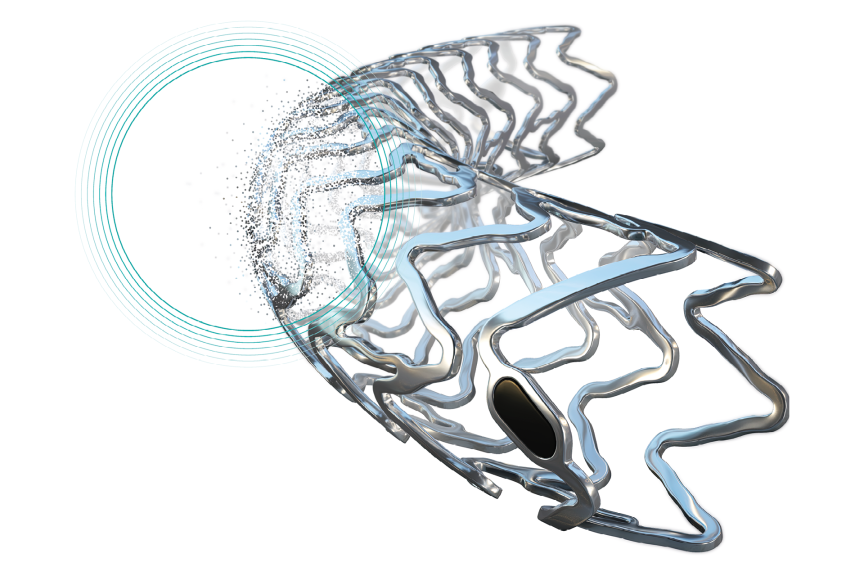 BUELACH, SWITZERLANDPress Release
BUELACH, SWITZERLANDPress ReleaseNew-Gen Resorbable Magnesium Scaffold Freesolve Enters BIOMAG-II Randomized Controlled Trial (RCT)
BIOTRONIK announced today the enrollment of the first patient in the BIOMAG-II trial aiming to evaluate the safety and clinical performance of its new-generation resorbable magnesium scaffold (RMS), Freesolve™, compared to a contemporary drug-eluting stent (DES). "We are delighted to enroll the first patient in the BIOMAG-II trial, which may play a critical role in helping establish resorbable metallic scaffolds as part of clinical practice in the future and I am particularly encouraged by the implantation results of the Freesolve resorbable magnesium scaffold," said Dr. Juan F. Iglesias, HUG
-
Image
 BÜLACH, SwitzerlandPress Release
BÜLACH, SwitzerlandPress ReleaseTwo-Year BIOPACT Randomized Controlled Trial (RCT) Analysis Demonstrates “Persistent Excellence” for Low-Profile Passeo-18 Lux DCB
BIOTRONIK announced the presentation of two-year results from the investigator-initiated BIOPACT RCT by Principal Investigator Dr. Koen Deloose at the Paris Vascular Insights 2023 congress. The randomized controlled non-inferiority trial evaluated the safety and efficacy of the Passeo®-18 Lux® drug-coated balloon (DCB) catheter compared to the In.Pact Admiral DCB (Medtronic), and showed excellent results for both balloons through 24 months. 1 The prospective, multicenter, core-lab adjudicated non-inferiority trial enrolled 302 patients in Austria, Belgium, France and Switzerland with
-
Image
 LAKE OSWEGO, Oregon, USAPress Release
LAKE OSWEGO, Oregon, USAPress ReleaseNewly Published Data Supports Effectiveness of BIOTRONIK Neuro’s RESONANCE™ Stimulation
Results from the BENEFIT-02 trial – the first of its kind to clinically evaluate a multiphase stimulation paradigm – support the effectiveness of RESONANCE multiphase stimulation used in the BIOTRONIK Neuro Prospera™ spinal cord stimulation (SCS) System in the treatment of patients with chronic pain. In contrast to other currently available SCS therapies, RESONANCE requires less power and uses a proprietary integrated circuit design to deliver a continuous, spatially and temporally distributed therapeutic pulse pattern across the spinal cord. Results of the study were recently published in
-
Image
 COPENHAGEN, DenmarkPress Release
COPENHAGEN, DenmarkPress ReleaseBIOLUX P-III BENELUX All-Comers Registry Demonstrates Safety and Efficacy of BIOTRONIK’s Drug-Coated Balloon in Isolated Popliteal Lesions at 24 Months
BIOTRONIK announced the two-year-results from the BIOLUX P-III BENELUX all-comers registry, presented by Principal Investigator Prof. Frank Vermassen at CIRSE 2023 in Copenhagen, Denmark. 1 The prospective, international, multicenter post-market registry evaluated the safety and efficacy of the Passeo ®-18 Lux ® drug-coated balloon (DCB) catheter in isolated popliteal artery lesions. This indication is considered a difficult vessel bed to treat due to its biomechanical constraints that usually preclude the placement of stents. BIOLUX P-III BENELUX registry enrolled 99 patients in Belgium, the
-
Image
 AMSTERDAM, The NetherlandsPress Release
AMSTERDAM, The NetherlandsPress ReleaseBIOMAG-I Study OCT Analysis Confirmed That BIOTRONIK’s Newest Resorbable Magnesium Scaffold Resorbed at 12 Months
New data from the BIOMAG-I first-in-human trial shed light on the vascular healing process following the implantation of DREAMS 3G, BIOTRONIK’s third-generation resorbable magnesium scaffold (RMS). A detailed intravascular optical coherence tomography (OCT) analysis demonstrated that 99.3% of the struts completely degraded at one year. 1 Dr. Masaru Seguchi from the German Heart Centre in Munich, Germany presented the findings at the European Society of Cardiology’s (ESC) congress in Amsterdam. While earlier BIOMAG-I study results provided favorable outcomes with regards to late lumen loss at
-
Image
 AMSTERDAM, The NetherlandsPress Release
AMSTERDAM, The NetherlandsPress Release12-Month-Data of BIOFLOW-DAPT Study Show Positive Results for Orsiro Mission DES With Short DAPT
BIOFLOW-DAPT one-year-data demonstrated non-inferiority and a good safety profile for the Orsiro ® Mission drug-eluting stent (DES) compared to Resolute Onyx DES (p<0.0001) in patients at high risk of bleeding receiving short dual antiplatelet therapy (DAPT). Prof. Marco Valgimigli presented the novel data in a late-breaking trial session at the European Society of Cardiology’s (ESC) Congress in Amsterdam. The results were published simultaneously in Circulation. BIOFLOW-DAPT is a prospective, multi-center, international, two-arm randomized controlled clinical study to assess the safety of one
-
Image
 BERLIN, GermanyPress Release
BERLIN, GermanyPress ReleaseFirst Implant of Amvia Sky in Europe, the World’s First Pacemaker Approved for LBBAP*
The first implant in Europe of BIOTRONIK’s latest pacemaker and CRT-P generation was conducted in early August by Prof. Dr. Jan De Pooter at the University Hospital Ghent in Belgium. The patient who received an Amvia Sky dual chamber pacemaker device was a 68-year-old man suffering from paroxysmal AV-block strongly limiting his physical efforts. “The implantation went on straightforward and very smoothly,” commented Prof. De Pooter after the procedure. “LBBAP enables pacing in a very natural, physiological way, benefiting the patient by offering physiological pacing and minimizing the risk for
-
Image
 BERLIN, GermanyPress Release
BERLIN, GermanyPress ReleaseAI-Supported Telemedicine Platform Aims to Improve the Health of Patients After Myocardial Infarction and Prevent Reinfarctions
The TIMELY consortium announced today the enrollment of the first patient in the randomized controlled clinical TIMELY trial. The TIMELY study investigates to what extent the AI-supported TIMELY platform improves the health of myocardial infarction (MI) patients and other patients with coronary heart disease after cardiac rehabilitation. Around 1.8 million people in the European Union die each year from coronary heart disease. 1 To prevent reinfarctions, patients receive cardiac rehabilitation to support necessary changes in lifestyle habits, such as diet and increased physical activity
-
Image
 BUELACH, SwitzerlandPress Release
BUELACH, SwitzerlandPress ReleaseBIOPACT RCT Subgroup Analyses Show Consistently Great Results for Passeo-18 Lux Drug-Coated Balloon
BIOTRONIK announced the one-year subgroup results from the investigator-initiated BIOPACT randomized controlled trial (RCT), which were presented by principal investigator Dr. Koen Deloose at LINC, the Leipzig Interventional Course 2023. The randomized controlled non-inferiority trial evaluated the safety and efficacy of the Passeo ®-18 Lux ® drug-coated balloon (DCB) catheter compared to the In.Pact Admiral DCB (Medtronic) and showed excellent results for both balloons through 12 months across a variety of sub-cohorts. The prospective, multicenter, core-lab adjudicated non-inferiority study
-
Image
 BUELACH, SwitzerlandPress Release
BUELACH, SwitzerlandPress ReleaseLate-Breaking Study Data: BIOTRONIK’s Orsiro DES Outperforms Other Ultrathin Strut Drug-Eluting Stent
In a late breaking trial session during EuroPCR 2023 in Paris, on behalf of the HOST-IDEA study investigators, Dr. Hyo-Soo Kim presented the results of a stent level analysis comparing two ultrathin strut drug-eluting stents (DES): Orsiro ® and Coroflex ISAR. The post-hoc comparison revealed significant differences in efficacy. HOST-IDEA is a large scale, multicenter, all-comers randomized controlled trial that demonstrated the non-inferiority of 3- to 6-month versus 12-month dual antiplatelet therapy (DAPT) after implantation of ultrathin strut DES. 2,173 patients in 37 South Korean centers
-
Image
 BERLIN, GermanyPress Release
BERLIN, GermanyPress ReleaseREACT DX Registry Highlights Relevance of Atrial High-Rate Episode Remote Monitoring in ICD Patients
A recent study highlights that implantable cardioverter-defibrillator (ICD) patients with new-onset atrial high-rate episodes (AHRE) often show high stroke risk while not being on oral anticoagulation (OAC), underlining the relevance of continuous AHRE burden monitoring. 1 The study also reports that the combination of BIOTRONIK’s unique DX ICD system with Home Monitoring is an effective and convenient way to detect AHRE. 1 REACT DX is a prospective, multi-center registry that enrolled 234 de novo ICD patients from 14 centers in Germany. It assessed the incidence of new-onset AHRE in an ICD
-
Image
 BUELACH, SwitzerlandPress Release
BUELACH, SwitzerlandPress ReleaseBIOTRONIK Launches Oscar Multifunctional Peripheral Catheter at LINC 2023
BIOTRONIK is pleased to announce the limited release of its Oscar ® ( One Solution: Cross. Adjust. Restore) multifunctional peripheral catheter and start of promotional activities at LINC, the Leipzig Interventional Course held June 6-9 in Leipzig, Germany. As indicated per Instructions for Use, the Oscar catheter is intended for dilation of stenotic segments in peripheral vessels. The device is comprised of three fully user-adjustable components (support catheter with integrated Lock Grip, extendable dilator and length-adjustable PTA balloon). It was developed to provide support during access
-
Image
 PARIS, FrancePress Release
PARIS, FrancePress ReleaseBIOMAG-I 12-Month Study Data Highlights Continued Excellent Patient Outcomes With New DREAMS 3G Scaffold
In the first-in-human study BIOMAG-I, BIOTRONIK’s new-generation DREAMS 3G resorbable magnesium scaffold (RMS) showed significantly lower in-scaffold late lumen loss (LLL) than its predecessor at 12 months as well as excellent safety and efficacy. Prof. Michael Haude, BIOMAG-I Coordinating Clinical Investigator, presented the latest results in the late breaking trial session at the EuroPCR course. 1 At one-year follow-up, BIOMAG-I data confirmed the excellent safety profile of DREAMS 3G RMS with a low target lesion failure rate of 2.6%. Neither cardiac death and myocardial infarction occurred
-
Image
 BERLIN, GermanyPress Release
BERLIN, GermanyPress ReleaseBIOTRONIK Receives CE Approval for the World’s First Pacemaker and CRT-P Family Approved for Left Bundle Branch Pacing*
BIOTRONIK, a leading global medical technology company with 60 years of experience in developing trusted cardiovascular and endovascular solutions, announced today the latest addition to its cardiac rhythm management portfolio. "We are excited to have received CE mark for our newest technology – the world’s first pacemakers and CRT-Ps approved for left bundle branch pacing. Amvia Sky and Amvia Edge represent cutting-edge innovation and incorporate the latest cardiology trends," says Dr. Andreas Hecker, President CRM/EP at BIOTRONIK. In 1963, BIOTRONIK introduced the first German implantable
-
Image
 BERLIN, GermanyPress Release
BERLIN, GermanyPress ReleaseFDA Approval for BIOTRONIK’s Prospera™ Spinal Cord Stimulation System
The company today announced U.S. Food and Drug Administration (FDA) approval for Prospera™, a spinal cord stimulation (SCS) system. The system features RESONANCE™, the first and only multiphase stimulation paradigm, paired with Embrace One™, a patient-centric care model that makes proactive care possible by offering automatic, objective, daily remote monitoring and ongoing management and support. 1 This approval marks the launch of the company’s new business segment, BIOTRONIK Neuro. Millions of people all over the world suffer from chronic intractable pain, a severe, constant, and
-
Image
 BERLIN, GermanyPress Release
BERLIN, GermanyPress ReleaseLa tecnología DX demuestra una alta precisión en la detección de la fibrilación auricular (FA) en pacientes con DAI monocameral
Los resultados del estudio MATRIX muestran que la alta precisión en la detección de los sistemas DAI DX con un solo cable para los episodios de FA (99,7 % para episodios ≥1 h), combinada con el potente rendimiento de transmisión de BIOTRONIK Home Monitoring ®, permite la monitorización remota fiable de la FA subclínica recomendada por las guías. Los resultados se publicaron en Europace a principios de esta semana. 1 MATRIX evaluó la utilidad del sistema DAI DX (detección de la señal auricular mediante un dípolo flotante integrado en el cable del DAI) para la monitorización remota de los
-
Image
 BUELACH, SwitzerlandPress Release
BUELACH, SwitzerlandPress ReleaseNew Data Highlight Promising Angiographic and Safety Profile of BIOTRONIK’s Third Generation Resorbable Magnesium Scaffold
Prof. Michael Haude, BIOMAG-I Coordinating Clinical Investigator, presented the latest results of the BIOMAG-I clinical study at the Cardiovascular Research Technologies (CRT) meeting. 1 At six months the angiographic and clinical data showed a low in-scaffold late lumen loss (LLL) rate and a good safety profile with no scaffold thrombosis. A low proportion of mal-apposed struts after implantation was observed, at six months struts were no longer discernable. The intravascular imaging documented a preservation of the scaffold area with a low mean neointimal area. Bioresorbable scaffolds have
-
Image
 BERLIN, GermanyPress Release
BERLIN, GermanyPress ReleaseBIOTRONIK Conduction System Pacing Tools Received CE Mark for Left Bundle Branch Area Pacing
BIOTRONIK announced today that it has received CE approval for the Selectra 3D implant tools to include left bundle branch area pacing (LBBAP) in addition to His-bundle pacing (HBP). Commonly referred to as conduction system pacing (CSP) these two approaches have emerged as a physiologic pacing alternative to avoid dyssynchronous contraction of the heart which can induce various negative long-term effects for patients. 1-3 Approved in 2021 for HBP, Selectra 3D’s safety and effectiveness for CSP has been shown in over 10 studies with more than 1,000 patients. In the multi-center Belgium
-
Image
 BUELACH, SwitzerlandPress Release
BUELACH, SwitzerlandPress ReleaseBIOTRONIK Announces Its Latest Innovation: Oscar Multifunctional Peripheral Catheter
BIOTRONIK announces the FDA 510(k) clearance and CE mark of its Oscar ® ( One Solution: Cross. Adjust. Restore) multifunctional peripheral catheter. Physicians in the U.S. have already used the novel device in more than 70 cases. As indicated per Instructions for Use, Oscar is intended for percutaneous transluminal interventions in the peripheral vasculature. The device was developed to provide support during access into and to dilate stenoses in femoral, popliteal and infrapopliteal arteries. The Oscar peripheral multifunctional catheter is comprised of three user-adjustable components: The
-
Image
 BERLIN, GermanyPress Release
BERLIN, GermanyPress ReleaseBIOTRONIK’s DX Technology Reaches Significant Milestone: 100,000 DX Devices Implanted
BIOTRONIK announced the milestone achievement of 100,000 implanted single-chamber implantable cardioverter-defibrillators (ICDs) equipped with DX Technology. Since the introduction of this unique technology in 2009, clinicians in more than 80 countries across all continents use DX ICDs. “Reaching this significant milestone, BIOTRONIK stands as a proud pioneer and partner in cardiac rhythm management. Our commitment to quality and focus on innovation sets us apart, allowing us to introduce meaningful innovations in the market, such as DX Technology, that make a real difference in patients’
-
Image
 BÜLACH, SwitzerlandPress Release
BÜLACH, SwitzerlandPress ReleaseBIOTRONIK and IMDS Join Forces to Launch Innovative Micro Rx Catheter
BIOTRONIK is proud to introduce the Micro Rx™ catheter, a novel rapid exchange microcatheter developed to easily enhance guidewire support during percutaneous coronary interventions (PCI). This cutting-edge device, exclusively distributed by BIOTRONIK, is manufactured by IMDS (Interventional Medical Device Solutions). Micro Rx catheter marks the fourth IMDS product BIOTRONIK has brought to the U.S., creating a compelling portfolio of devices which includes NHancer Rx, TrapIT, and ReCross catheters. The Micro Rx catheter features a reinforced distal shaft with a core wire between two layers of
-
Image
 BÜLACH, SwitzerlandPress Release
BÜLACH, SwitzerlandPress ReleaseCE-approval for BIOTRONIK’s Next-Gen Metallic Bioresorbable Scaffold Freesolve
BIOTRONIK announces the CE approval and launch of Freesolve™ Resorbable Magnesium Scaffold (RMS). This third generation RMS has been engineered to provide optimized vessel support, yet achieves magnesium resorption within 12 months. 1 The new Freesolve RMS is a groundbreaking vascular advancement based on reliable clinical evidence. Recent BIOMAG-I trial data highlights an exceptional 99.3% magnesium strut degradation 12 months after implantation 2, consistent performance, regardless of lesion characteristics, and restoration of vasomotion. 3 "Having closely observed the evolution of
-
Image
 SINGAPOREPress Release
SINGAPOREPress ReleaseBIOTRONIK Opens New Asia-Pacific Hub in Singapore
BIOTRONIK announced today the opening of its new Asia Pacific Manufacturing and Research Hub. The 20,000 m² site will serve as the company’s central hub in Asia-Pacific, with hundreds of employees working in manufacturing, quality, research & development (R&D), and sales and marketing. BIOTRONIK, founded in Berlin, Germany, in 1963 is a pioneer in implantable pacemakers, defibrillators, and vascular intervention for 60 years. BIOTRONIK’s presence in Singapore began in 2012, and in 2016, the company inaugurated its first manufacturing site, complementing its high-quality manufacturing sites in
-
Image
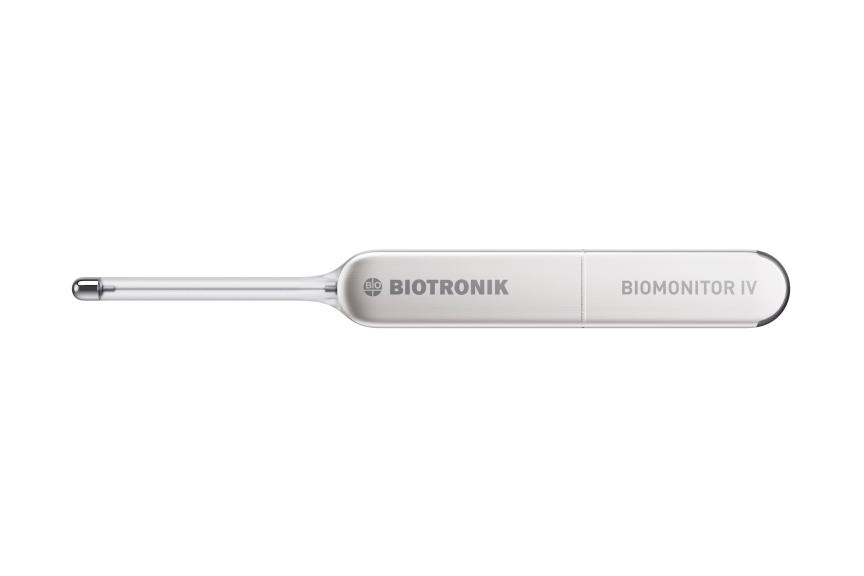 BERLIN, GermanyPress Release
BERLIN, GermanyPress ReleaseBIOTRONIK Introduces New BIOMONITOR IV Insertable Cardiac Monitor with Artificial Intelligence at EHRA Congress 2024
BIOTRONIK announced the CE approval and first European implant of its latest insertable cardiac monitor (ICM). The BIOMONITOR IV features artificial intelligence (AI) for false positive reduction. It is the only ICM on the market with premature ventricular and atrial contraction (PVC and PAC) discrimination capabilities, 1,2 as well as industry-leading signal quality and transmission success for highly reliable remote patient management. 3 False positive detections may take clinicians a considerable amount of time to review. That’s why BIOMONITOR IV features SmartECG, an intelligent system
-
Image
 LAKE OSWEGO, Oregon, USAPress Release
LAKE OSWEGO, Oregon, USAPress ReleaseBENEFIT-01 Study Now Published in Neuromodulation: A Glimpse into the Future of Spinal Cord Stimulation Programming
BIOTRONIK is pleased to announce the release of the results of the BENEFIT-01 study published in Neuromodulation. 1 The prospective, multi-center study was designed to investigate the influence of clinically relevant Spinal Cord Stimulation (SCS) parameters on patient perception. BENEFIT-01 marked the beginning of a series of SCS clinical studies with the aim of developing an SCS technology that provides sustained pain relief with few side effects. From July to November 2017, 40 patients with low back and/or leg pain who had completed a commercial SCS trial were enrolled at seven clinical
-
Image
 BERLIN, GermanyPress Release
BERLIN, GermanyPress ReleaseBIOTRONIK Introduces World’s First and Only System CE Approved for Left Bundle Branch Area Pacing (LBBAP)
Following the recent CE approval of the Solia S lead 1 for LBBAP, BIOTRONIK proudly announces the world’s first and only complete CSP system, now fully CE-approved for Left Bundle Branch Area Pacing (LBBAP). 2 The BIOTRONIK CSP system comprises three proven components: Selectra 3D catheters offering exceptional handling 3, Solia S leads – the most popular choice of stylet-driven leads for LBBAP 4 providing enhanced stability 3, and the Amvia pacemaker family, the first and only family approved for LBBAP. 2 Notably, this groundbreaking system is the world’s first and only approved MR
-
Image
 BERLIN, GermanyPress Release
BERLIN, GermanyPress ReleaseNew BIOlMASTER.Selectra 3D Study Highlights Exceptional Safety and Performance of BIOTRONIK’s Selectra 3D Guiding Catheter for CSP
In a recent prospective, international, multicenter, nonrandomized trial, the BIO|MASTER.Selectra 3D study assessed the performance and safety of BIOTRONIK’s Selectra 3D guiding catheter, revealing compelling insights into its ease of handling and effectiveness. Conducted across 10 centers in Australia, Hong Kong and Europe, the study included 157 patients who underwent a CSP procedure for bradycardia pacing or heart failure indications. The results of this research have been published in the Heart Rhythm O 2 journal. 1 Key Findings of the BIO|MASTER.Selectra 3D Study: High Success Rate in
-
Image
 BÜLACH, SwitzerlandPress Release
BÜLACH, SwitzerlandPress ReleaseFDA Breakthrough Device Designation for BIOTRONIK Freesolve™ Below-the-Knee Resorbable Magnesium Scaffold (RMS)
BIOTRONIK has been granted Breakthrough Device Designation (BDD) from the US Food and Drug Administration (FDA) for the Freesolve™ below-the-knee resorbable magnesium scaffold (BTK RMS). The Freesolve BTK RMS is designed for individuals suffering from chronic limb-threatening ischemia (CLTI). CLTI is the most severe form of peripheral arterial disease (PAD), estimated to affect 11% of the 200 million people suffering from PAD globally. CLTI is associated with high rates of amputation and mortality as well as high care costs. 1 To qualify for a Breakthrough Device Designation, a device
-
Image
 LAKE OSWEGO, Oregon, USAPress Release
LAKE OSWEGO, Oregon, USAPress ReleaseThe Future of Single Chamber ICDs: BIOTRONIK Takes Unprecedented Market Stance with Proprietary DX Technology
BIOTRONIK, a leader in implantable medical device technology, announced today they will solely supply their proprietary DX models for new single-chamber ICD implants moving forward. The move is being made in response to overwhelming recent clinical data demonstrating superior diagnostics and decreased complication risk of DX compared to traditional high-voltage systems. 1-4 “Based on the current evidence, it is clear we must focus on what is proving to be a lower risk technology with added benefits,” said Dr. Alexander Uhl, BIOTRONIK CEO. “Therefore, we have made the decision to offer only DX
