Key Results
Key Result 1
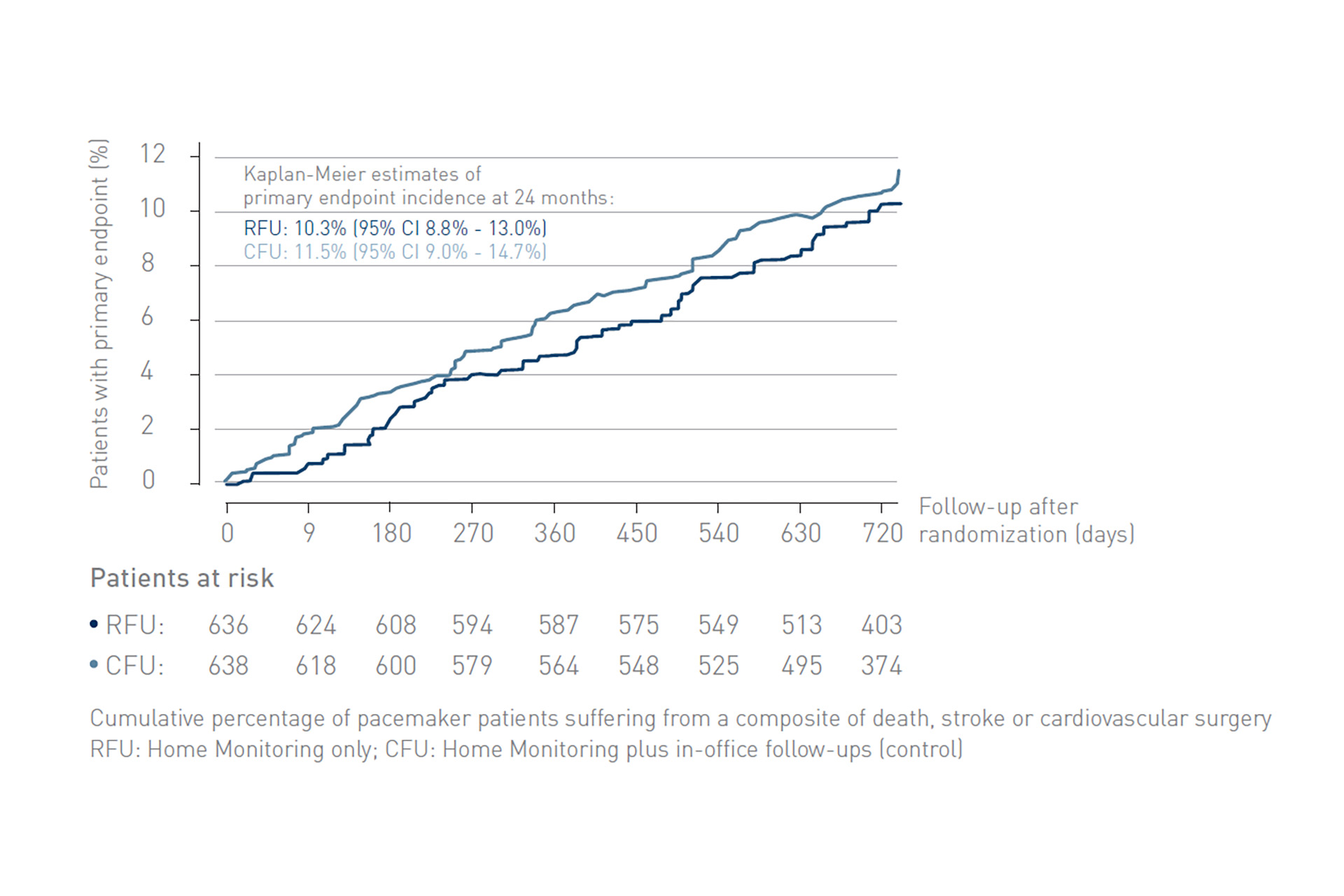
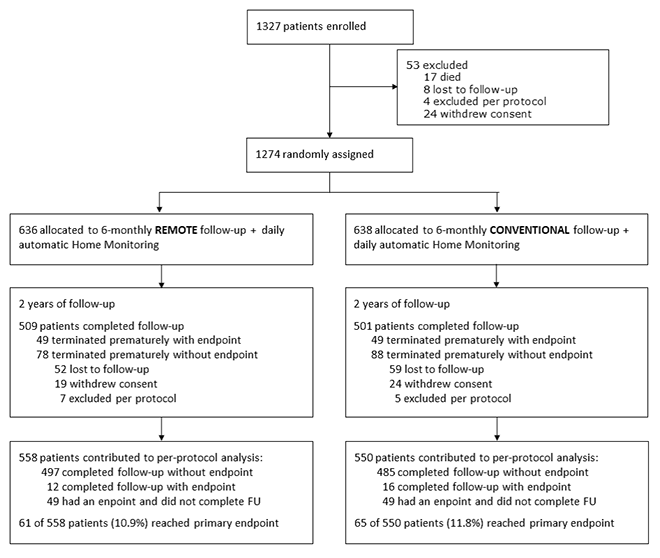
The At-Home study demonstrated non-inferiority of exclusive remote follow-up over 2 years in comparison to continuous remote monitoring associated with in-office follow-ups for real-world pacemaker patients.
Figure 1: Kaplan-Meier curves of incidence of primary endpoints (a composite of death, stroke, or cardiovascular surgical procedure), starting at randomization. RFU, remote follow-up; CFU, conventional follow-up.