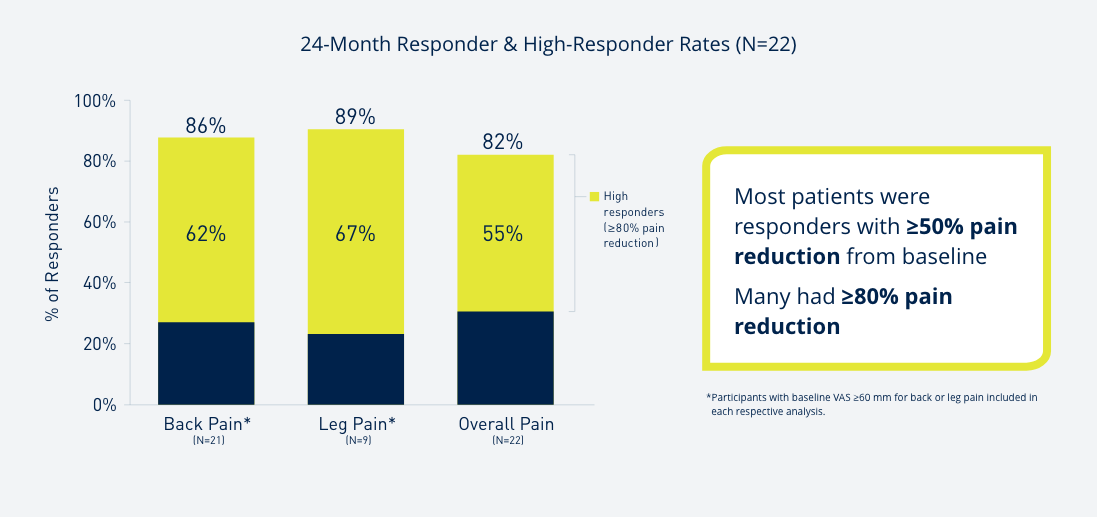
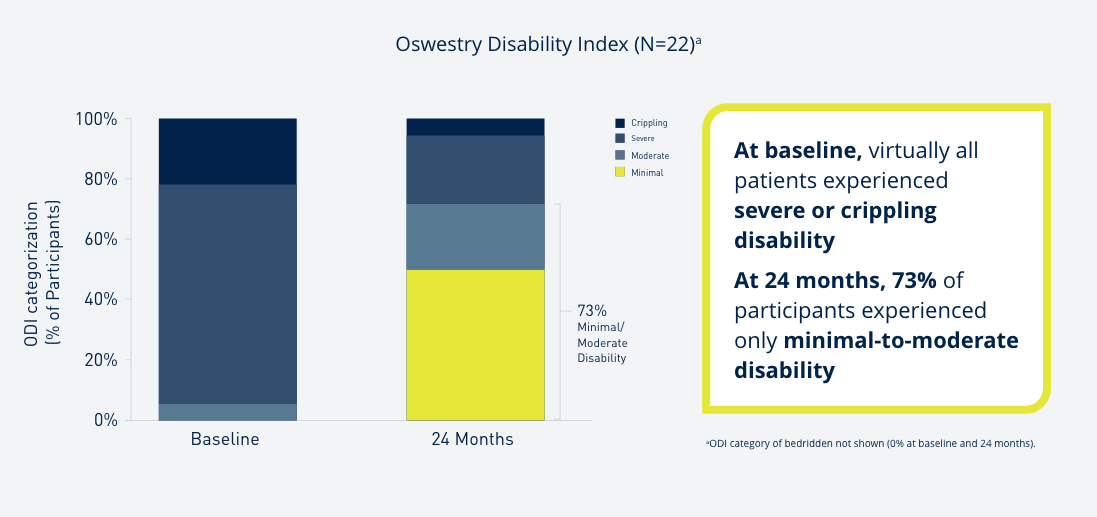
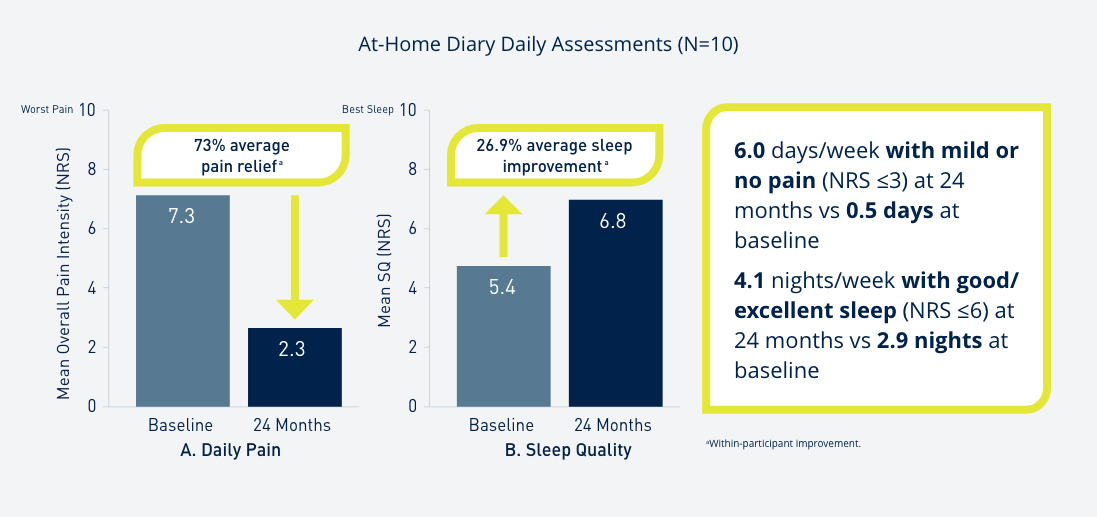
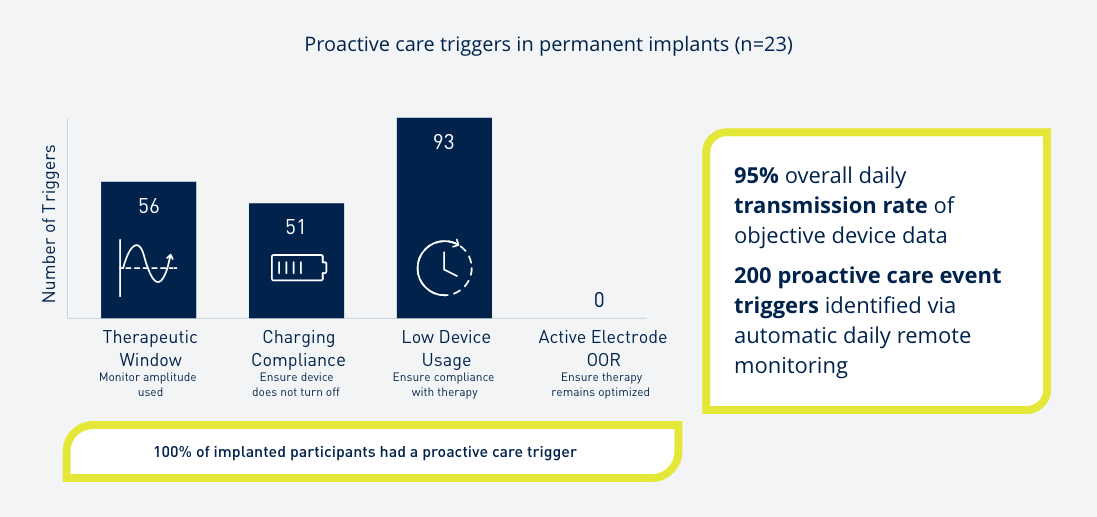
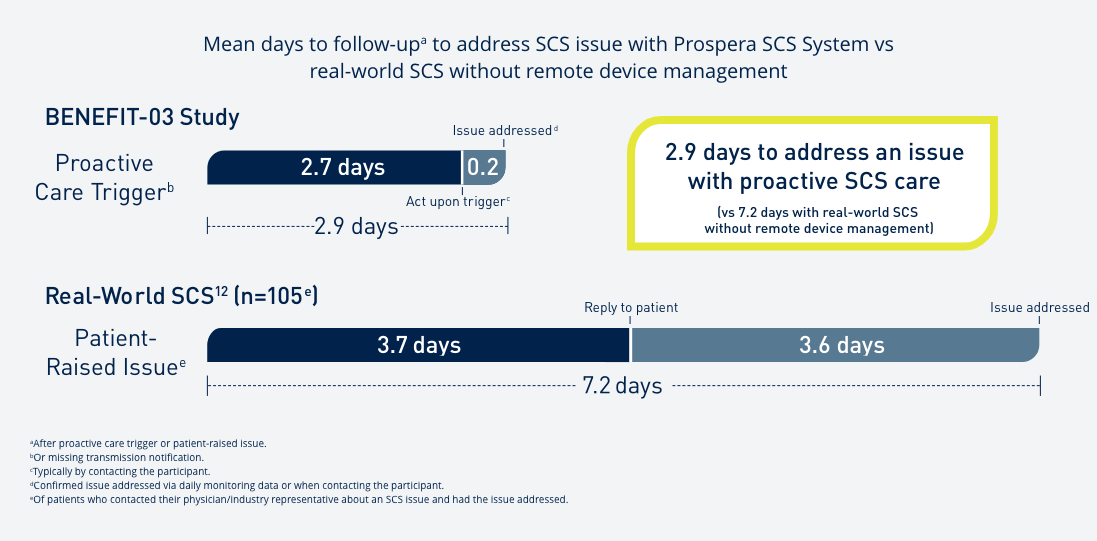
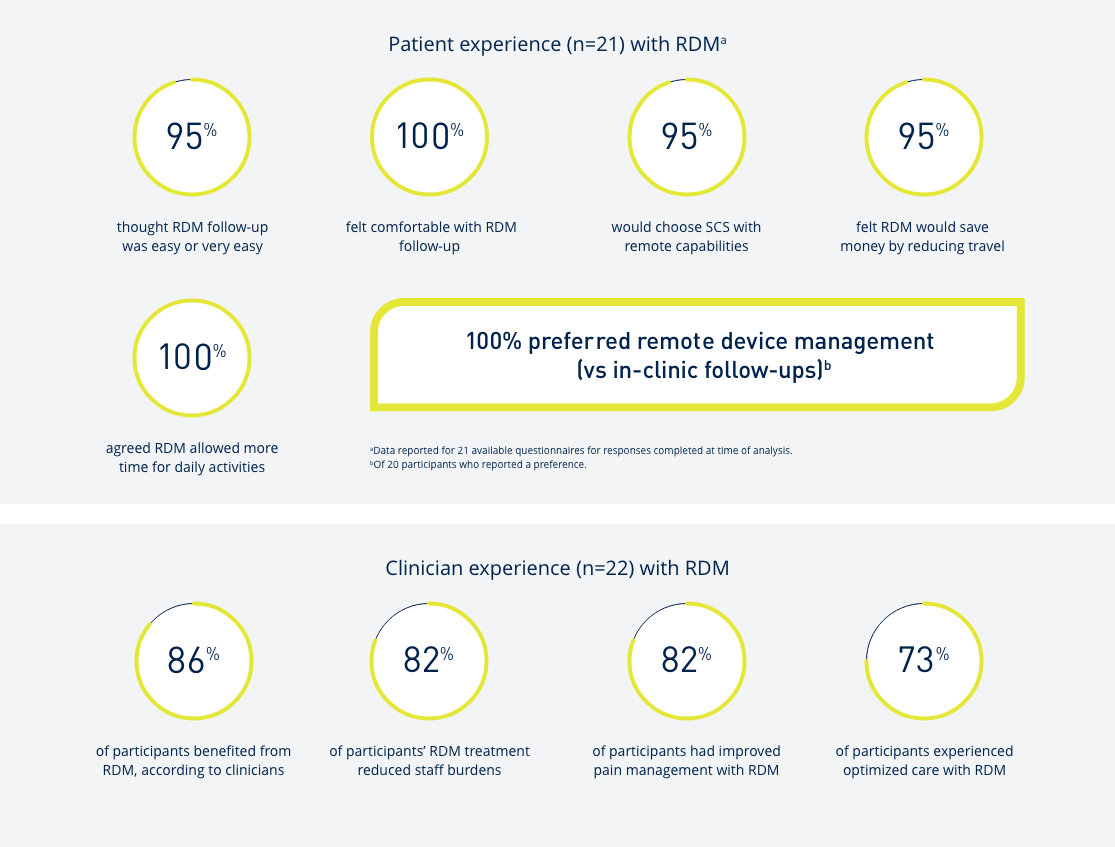
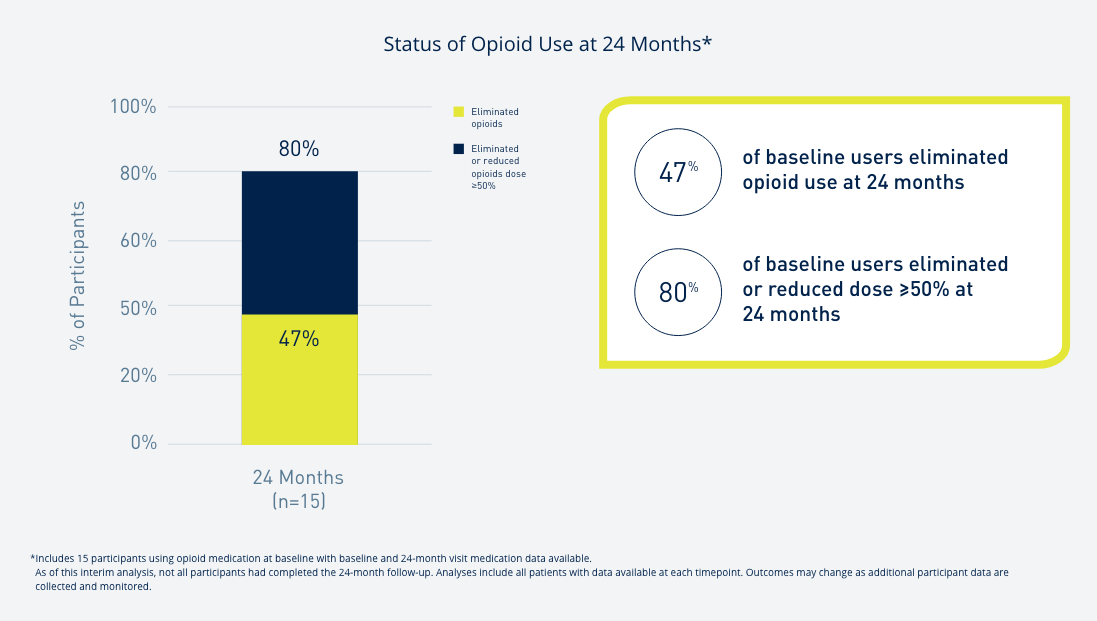
Introduce yourself to Prospera Spinal Cord Stimulation with Embrace One.
Through automatic, objective, daily remote monitoring of the device and therapy, we put true proactive care* into action so that the therapy you prescribe remains optimized for each patient, every day, over the lifetime of the therapy.
Watch the video to learn more.