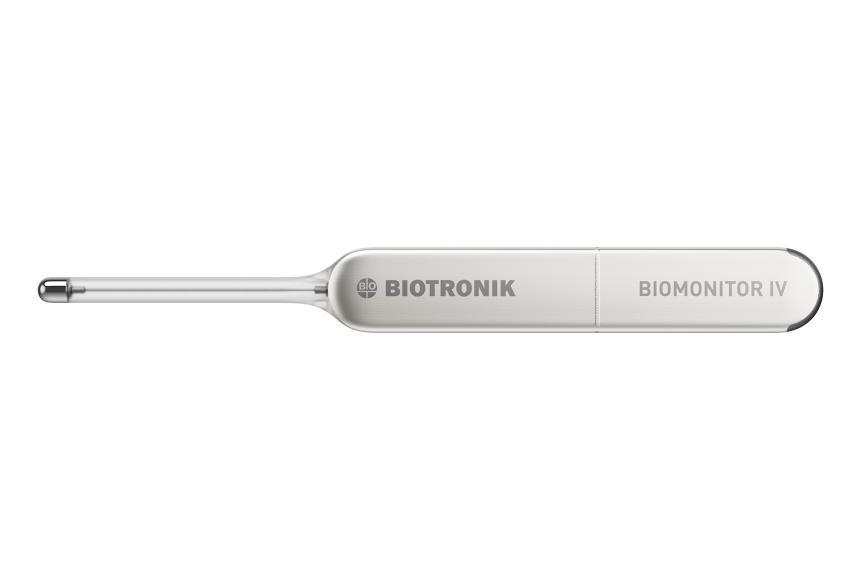
BIOMONITOR IV
A real challenge exists in the clinical world today with the volume of data generated as more patients benefit from the proven value of long-term cardiac monitoring. Valuable time and resources are spent analyzing false positive arrhythmias, leaving patients waiting for accurate answers. The intelligent technology in BIOMONITOR IV can help.
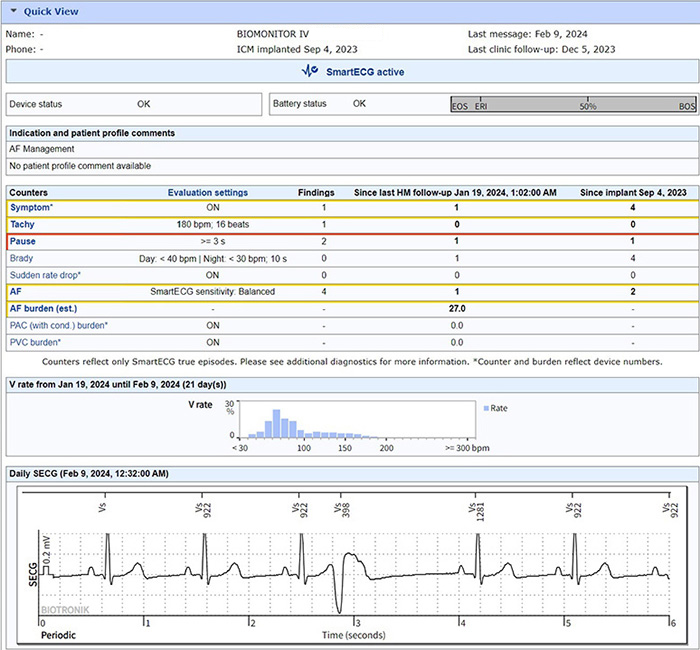
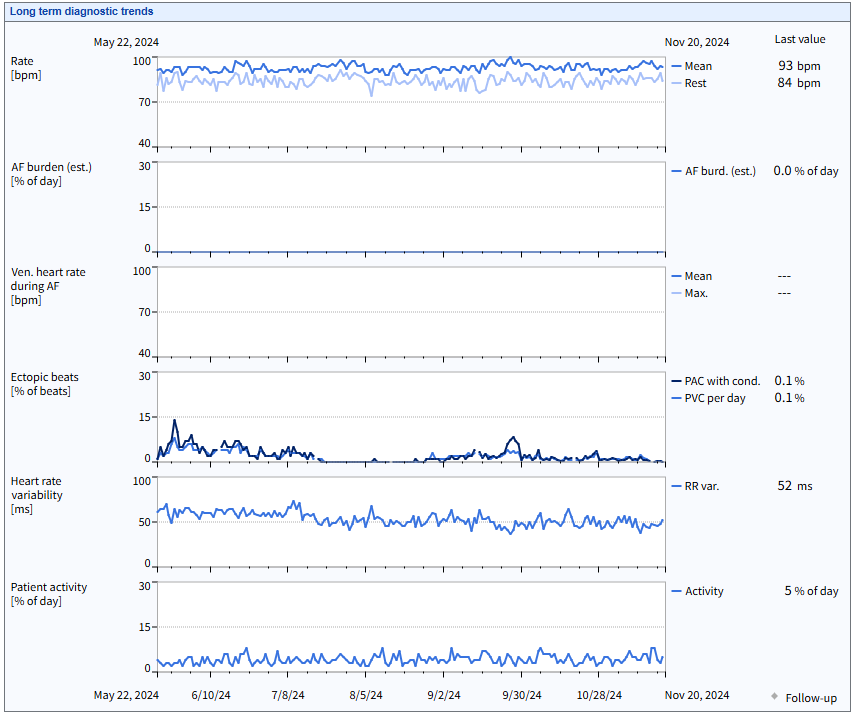
BIOMONITOR IV with SmartECG leverages artificial intelligence (for AF only) and advanced detection algorithms to reduce 86% of false positives across all major arrhythmias,1 alleviating the review burden and helping clinicians achieve timely and accurate diagnosis to focus on what’s most important – the patient.
Product Highlights
Intelligent SmartECG
AI* and advanced detection algorithms reduce 86% of false positives across all major arrhythmias1 to alleviate the review burden4
Confidence from BIOTRONIK Home Monitoring
Streamlined ICM monitoring with indication-specific templates, enhanced automaticity, and intuitive reports
Clarity with PVC/PAC Diagnostics
The industry’s first to discriminate between PVCs and PACs and clearly report burden over time for personalized treatment decisions
Product Overview

Fractal iridium coating
Flexible silicone antenna
BIOvector for industry-leading signal quality
Titanium can
5 years longevity13
Fractal iridium coating
Media
Downloads & Related Links
Contact Us
References
*For AF Only
1. Data on file. With AF filtering criterion set to Very Specific in the Home Monitoring Service Center. Reduction is relative to performance without SmartECG.; 2. Iwai et al., Novel automated overread strategy significantly improves clinical workflow in ICM remote monitoring. Presented at 16th Asia Pacific Heart Rhythm Society Scientific Session in Conjunction with CardioRhythm (APHRS 2023), 31 August - 3 September, Hong Kong; 3. Bisignani et al., Implantable cardiac monitors: Artificial intelligence and signal processing reduce remote ECG review workload and preserve arrhythmia detection sensitivity. Frontiers in Cardiovascular Medicine. Volume 11 - 2024. doi: 10.3389/fcvm.2024.1343424; 4. Mariani JA, Weerasooriya R, van den Brink O, Mohamed U, Gould PA, Pathak RK, et al. Miniaturized implantable cardiac monitor with a long sensing vector (BIOMONITOR III): Insertion procedure assessment, sensing performance, and home monitoring transmission success. J Electrocardiol. 2020;60:118–25. doi: 10.1016/j.jelectrocard.2020.04.004; 5. Dukes et al., Novel PVC detection algorithm in an implantable cardiac monitor for longitudinal risk monitoring. Presented at ESC Congress 2023, August 25-27; Amsterdam, Netherlands; 6. Marmerstein, Joseph, et al. Evaluation of a Novel PVC and PAC Detection Algorithm in an Implantable Cardiac Monitor for Longitudinal Risk Monitoring. Heart Rhythm O2, Aug. 2023; 7. Binici Z, Intzilakis T, Nielsen OW, Køber L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121(17):1904–11. doi: 10.1161/CIRCULATIONAHA.109.874982; 8. Larsen BS, Kumarathurai P, Falkenberg J, Nielsen OW, Sajadieh A. Excessive Atrial Ectopy and Short Atrial Runs Increase the Risk of Stroke Beyond Incident Atrial Fibrillation. J Am Coll Cardiol. 2015;66(3):232–41. doi: 10.1016/j.jacc.2015.05.018; 9. Todo K, Iwata T, Doijiri R, Yamagami H, Morimoto M, Hashimoto T, et al. Frequent Premature Atrial Contractions in Cryptogenic Stroke Predict Atrial Fibrillation Detection with Insertable Cardiac Monitoring. Cerebrovasc Dis. 2020:1–7. doi: 10.1159/000505958. PubMed PMID: 32023609; 10. Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, et al. Ventricular Ectopy as a Predictor of Heart Failure and Death. J Am Coll Cardiol. 2015;66(2):101–9. doi: 10.1016/j.jacc.2015.04.062. 11. Mariani JA et al. J Electrocardiol. 2020, 60; 12. Deneke et al VOLUME 3, ISSUE 2, P152-159, APRIL 01, 2022, https://www.heartrhythmopen.com/article/S2666-5018(22)00044-7 13. 6-month storage period; Sensing: 60 bpm; daily device message via Home Monitoring including one automatic subcutaneous ECG per day and 2 patient-triggered subcutaneous ECGs per month. See Technical Manual for more details.
Indications for use: The BIOMONITOR IV is indicated to detect the following cardiac arrhythmias:
- Atrial fibrillation
- Bradycardia
- Sudden Rate Drop
- Tachycardia
- Pause
Contraindications: There are no known contraindications for the insertion of the BIOMONITOR device.
Warnings and Precautions: Reference applicable technical manuals for detailed information about other devices used with the BIOMONITOR device. Certain therapeutic and diagnostic procedures may cause undetected damage to an insertable cardiac monitor (ICM), resulting in malfunction or failure at a later time. Please note the following warnings and precautions:
MR Conditional - The cardiac monitor is labeled as MR conditional. Conditions for MR scans of the BIOMONITOR device are located in the appropriate technical manual.
Storage (temperature) - Recommended storage temperature range is -10° to 45°C (14°-113°F). Exposure to temperatures outside this range may result in insertable cardiac monitor malfunction.
FOR SINGLE USE ONLY - Do not resterilize the insertable cardiac monitor, incision tool or insertion tool; they are intended for one-time use.
Use Before Date - Do not implant the device after the USE BEFORE DATE because the device sterility and longevity may be compromised.
Sharp - Packaging includes an incision tool that is sharp and should be handled with care.
For additional information see instructions for use at manuals.biotronik.com.