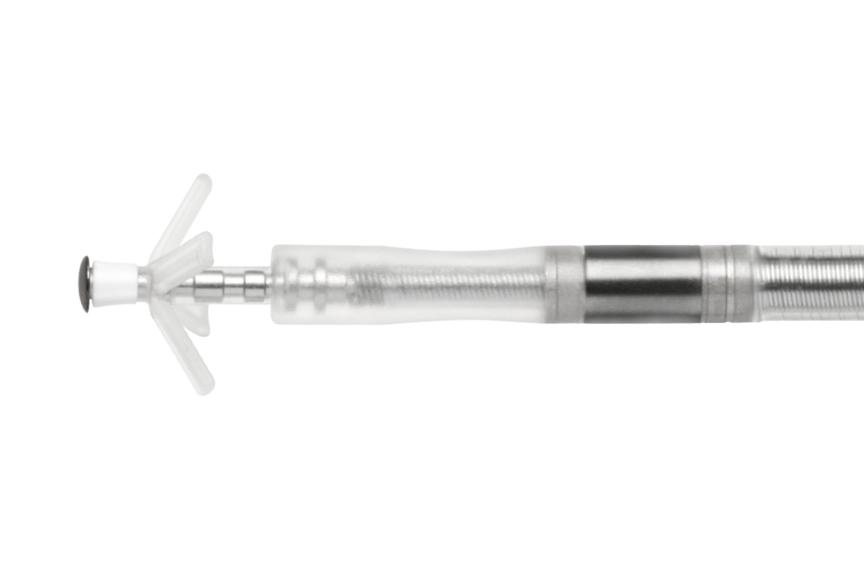
Solia S
With more than 3 million implants worldwide,1 the Solia family is a complete portfolio of MR conditional active and passive fixation pacing leads. The Solia S lead is the only FDA-approved LBBAP lead backed by a prospective IDE clinical trial.2
The Solia family of pacing leads offers 5.6 F lead body diameter (6 F introducer) that is MR conditional, together with a polyurethane sleeve over a silicon insulator designed for reduced friction. Solia leads are approved for use with all BIOTRONIK ProMRI systems, giving physicians more options to accommodate varying patient anatomies.
Product Highlights
FDA Approved for LBBAP
The Solia S lead is the only FDA approved LBBAP lead backed by a prospective IDE clinical trial.
Implant with Confidence
Low lead complication rate, high implant success rate and low, stable threshold at 12-month follow-up in LBBAP implants3,4
Drive Efficiency
17% shorter lead implantation time for stylet-driven leads versus lumenless leads in LBBAP procedures.5
Product Details
- 5.6F lead body
- Stylet-driven
- Extendable/retractable helix
- 10 mm distal collar-ring spacing
- Polyurethane coating over silicone designed to reduce friction
- 45 cm, 53 cm, and 60 cm lengths
- Only lead to be FDA approved for LBBAP following a prospective clinical trial (BIO-CONDUCT, 2024)*
- Unique BIOTRONIK fractal coating enlarges the bioeffective surface area for improved pacing and sensing6
LBBAP Lead Delivery System
Facilitating Physiologic Pacing For Your Patients
Used in more than 80,000 patients worldwide and backed by more than 30 clinical studies to date,7 BIOTRONIK's Solia S lead and Selectra 3D catheter are the first and only FDA approved stylet-driven lead and dedicated delivery catheter combination for LBBAP.
- Solia S is the only lead backed by a prospective IDE trial, demonstrating a high implant success rate of 95.7%.3
- 17% shorter lead implantation time for stylet-driven leads vs. lumenless leads5
- Less hassle with continuous electrical measurements and no need for special, costly tools.8
*45 cm length not approved for LBBAP
Downloads & Related Links
Contact Us
References
1. Data on file. As of December 2024.
2. BIOTRONIK Solia S technical manual. Abbott Ultipace, Tendril Lead technical manuals. Boston Scientific Ingevity+ lead technical manual. Medtronic SelectSecure 3830 technical manual.
3. Liu, et al. Left bundle branch area pacing using a stylet-driven, retractable-helix lead: Short-term results from a prospective multicenter IDE trial (the BIO-CONDUCT study). Heart Rhythm. 2024 May 19:S1547-5271(24)02547-5. Epub ahead of print.
4. De Pooter, et al. Initial experience of left bundle branch area pacing using stylet-driven pacing leads: A multicenter study. J Cardiovasc Electrophysiol. 2022; 33(7):1540-1549.
5. Sritharan, et al. Procedural outcome and follow-up of stylet-driven leads compared with lumenless leads for left bundle branch area pacing. Europace. 2023 Oct 5;25(10)
6. Data on file.
7. Data on file. As of July 2024.
8. Burri H, Jastrzebski M, Cano Ó, et al. EHRA clinical consensus statement on conduction system pacing implantation: endorsed by the Asia Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS), and Latin American Heart Rhythm Society (LAHRS). Europace. 2023; 25(4): 1208-1236.