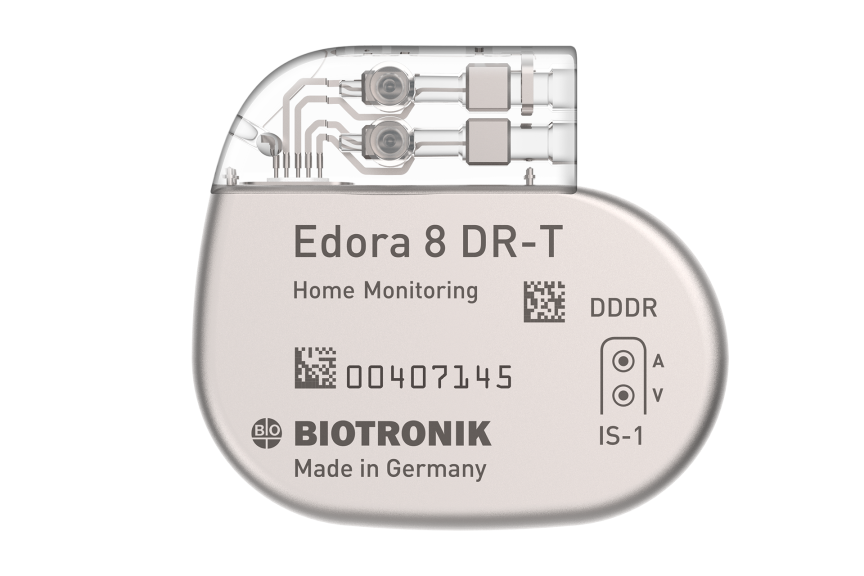
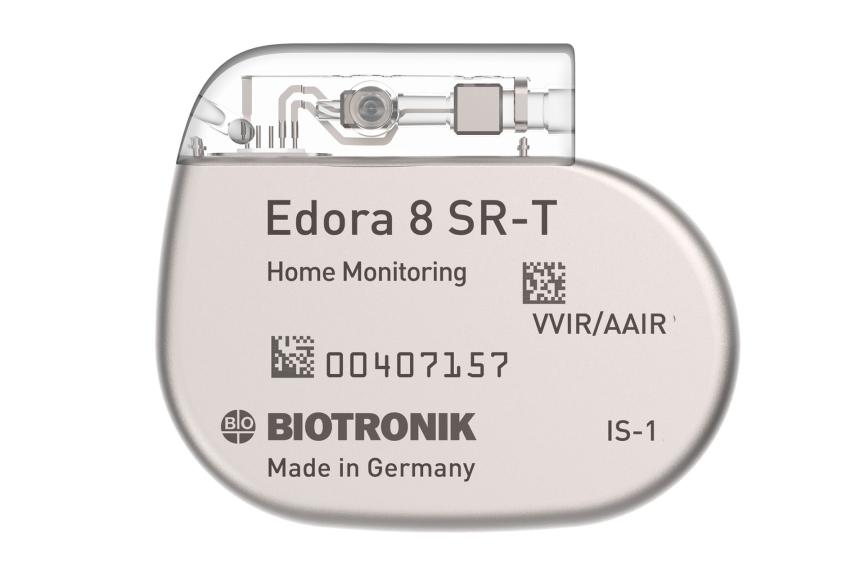
Edora 8 DR-T/SR-T
Edora is one of the smallest1 full-featured MRI pacemaker in the US. Compared to its predecessor, Edora’s reduced form factor has no negative effects on its battery, still boasting an impressive 13 years2 of longevity in the single-chamber variant. Consistent with BIOTRONIK’s long-standing MRI leadership, Edora offers MRI AutoDetect, a revolutionary feature that is designed to improve efficiency for providers by eliminating the post-scan reprogramming requirement. It also aims to improve the patient experience by reducing the length of time the device is in MRI mode and the added benefit of flexibility in scheduling.
Product Highlights
Small Size
Improves patients’ comfort through a reduced device volume.
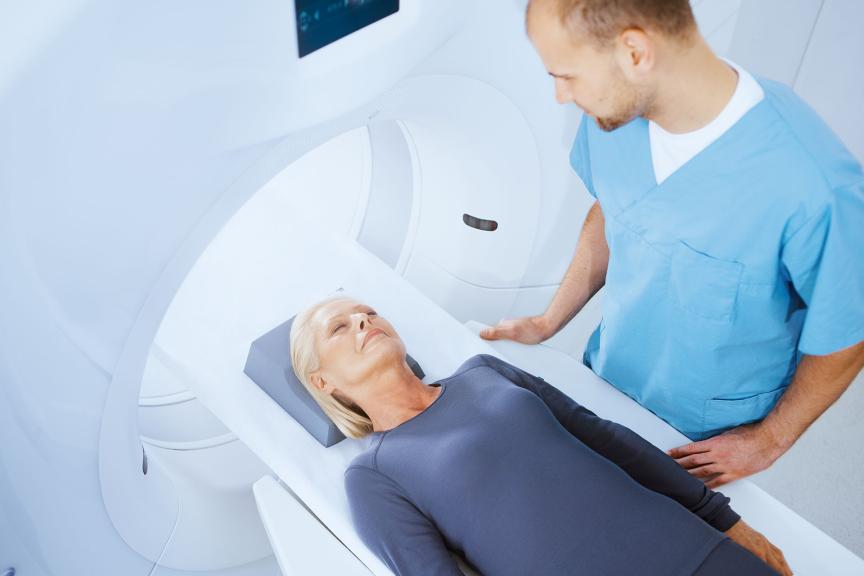
Simplifying MRI Access
MRI AutoDetect — Simplifies workflows through automatic detection of MRI environment and minimizes patients’ time in MRI mode.
The Market's Only Myocardial Contractility Measurement
CLS: Translates changes in myocardial impedance to pacing rate changes based on metabolic demand. Now programmable with up to 30 ms in V-to-V offset in CRT-P patients.
Product Details
| Small Size Improves patients’ comfort through a reduced device volume. |
BIOTRONIK Home Monitoring® Effective remote monitoring of heart failure and system integrity based on automatic and wireless daily transmissions. |
| MRI AutoDetect Simplifies workflows through automatic detection of MRI environment and minimizes patients’ time in MRI mode. |
Capture Control Extends device longevity by automatically adjusting the pacing amplitudes. |
| Closed-Loop Stimulation (CLS) The only rate adaptation algorithm that is FDA-approved to respond to physiologic demands and acute mental stress, on a beat-to-beat basis. |
SafeSync RF Telemetry RF telemetry for wandless, time-saving and reliable data transmission at implantation and follow-up. |
| ProMRI®3 Allows patients to undergo MR scanning under specific conditions |
Event-Triggered Wireless IEGM Transmissions Within 24 Hours Enable prompt evaluations for fast and better informed therapy decisions. |
Manual
Product Manual
Media
Contact Us
References
- 10 cc SR-T volume 2. AAI(R) @ 2.5 V, 0.4 ms, 60 ppm, 100% pacing, 500 Ω, RF-Telemetry off. 3. For combination of MR conditional devices, please see the "ProMRI® MR conditional device systems" manual.