BIO-LIBRA Initial Interim Analysis Results Demonstrate Higher Probability of Ventricular Tachyarrhythmias or Mortality in Men than Women Poster presented sharing first results from study designed with emphasis on both sex and device specific factors sees significant differences
WASHINGTON, United States, April 4, 2022 – In conjunction with BIOTRONIK, Valentina Kutyifa, MD, PhD, presented the first interim analysis results from the BIO-LIBRA study in a poster contribution at the American College of Cardiology’s 71st Annual Scientific Session.
Women have been traditionally underrepresented in clinical trials conducted in patients with implanted medical devices, contributing to only 20-25 percent of the study population, significantly limiting our understanding of sex-specific outcomes. In a quest to close this gap, Valentina Kutyifa, MD, PhD, Jeanne Poole, MD, and BIOTRONIK designed and conducted the BIO-LIBRA study to ensure that men and women are equally represented.
The results presented today show significant differences in one-year ventricular tachyarrhythmia or death rates by sex in patients with non-ischemic cardiomyopathy with an ICD or CRT-D. The one-year probability of VT/VF or death was 7% in women and 13% in men, higher than expected by the study investigators.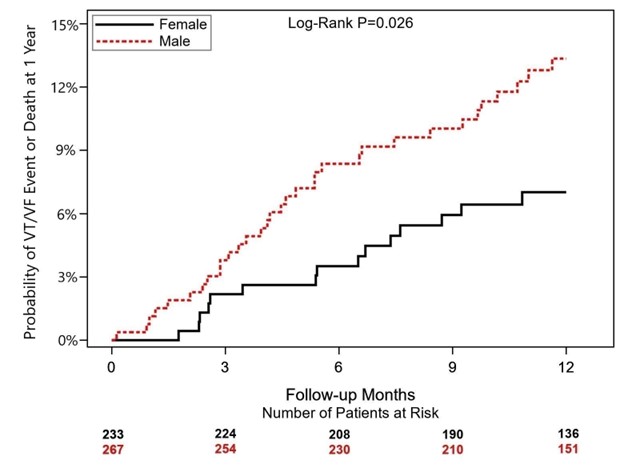
Figure: One-Year Cumulative Probability of Ventricular Tachyarrhythmias or Death by Sex in the BIO-LIBRA Study
The BIO-LIBRA study, launched in 2019, is a multi-center, prospective, observational registry study designed to improve our understanding of outcomes in men and women with non-ischemic cardiomyopathy and an implanted ICD or CRT-D, while also observing demographic differences. The study has enrolled 1,000 patients with non-ischemic cardiomyopathy with an ICD or CRT-D at 48 centers through the United States. The primary endpoint of BIO-LIBRA is ventricular tachyarrhythmia or death.
BIO-LIBRA has been designed to specifically encourage a minimum of 40% female enrollment as a way of addressing the limited contemporary data available on sex-specific outcomes as related to this type of treatment.
The results announced today include the study’s initial 500 patients with a completed 1 year of follow-up. This population is 47% female, which exceeds stated goals, and 37% of the subjects are non-white, truly representing a diverse population more representative of the real world.
Further results will be presented as the study progresses.
-END-
Kutyifa V presented at ACC.22 - Poster Contribution April 4, 2022, Session 1558 - Heart Failure and Cardiomyopathies
About BIOTRONIK:
At BIOTRONIK, patient well-being is our top priority and has been for 60 years. BIOTRONIK is a leading global medical technology company with products and services that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation solutions. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries.