Late-breaking trial presented at HRS shows measurable difference in outcomes between patients by sex BIOTRONIK continues to champion diversity in clinical trial enrollment with ongoing BIO-LIBRA Study
LAKE OSWEGO, Ore., United States – Data presented at Heart Rhythm Society’s 2023 Conference show a measurable difference in outcomes between male and female patients when looking at one-year ventricular tachyarrhythmia or death rates in patients with non-ischemic cardiomyopathy (NICM) with an ICD or CRT-D.
Historically, women have been underrepresented in studies of patients with implantable cardiovascular medical devices, making it impossible to draw measurable conclusions of differences between sex. In recognition of this disparity, BIO-LIBRA was purposefully designed by Valentina Kutyifa, MD, PhD, and Jeanne Poole, MD, in collaboration with BIOTRONIK and the University of Rochester Data Coordinating Center to begin to close that research gap with an intentionally diverse patient population.
“The BIO-LIBRA model will lead the way women are enrolled in medical device studies in the next decades,” says Dr. Valentina Kutyifa. “A large, properly powered study was much needed to better understand sex-specific outcomes in non-ischemic cardiomyopathy patients, and the BIO-LIBRA study sheds new light on the intricate interconnected relationship between sex and race on outcomes.”
Data shown in a late-breaking presentation included 1-year data for the full 1000 patient cohort, in which 48% are female and 30% are non-white, a first of its kind for studies of this nature.1 Results showed that women with NICM, especially those with a CRT-D, were found to experience a significantly lower rate of arrhythmias or death during follow-up. Significant interaction was also observed between sex and device type.
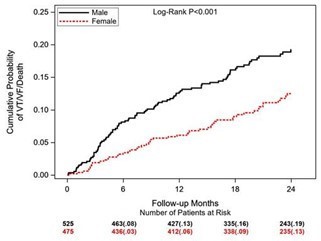
BIO-LIBRA: Primary Objective: Ventricular Arrhythmias or Death by Sex1
In addition, the results of a BIO-LIBRA pre-specified sub-group analysis exploring outcomes by race in the first 500 patient cohort was presented at HRS.2
This commitment to increasing diversity in research comes from a mission to provide the highest quality care for all patients, as well as an awareness that creating a company culture where diversity, equity and inclusion are a part of life allows all employees to succeed to their highest potential.
“At BIOTRONIK, we are keenly aware of the importance and value of increasing diversity and equal access to inclusion in clinical trials, to in turn generate representative clinical evidence for our patients, physicians and the medical community,” says Amy Culley, VP of Clinical Studies, BIOTRONIK, Inc. “As a result of that awareness, we strive to live our commitment to those values in our company culture and in our community through the body of research that we support.”
The BIO-LIBRA study was launched in 2019, and is a multi-center, prospective, observational registry study which includes 1000 patients in 48 centers throughout the United States. Patients will be followed for three years, and final results will be presented after study completion.
-END-
References:
1. Kutyifa V, et al. Contemporary Outcomes of Non-Ischemic Cardiomyopathy Patients with an Implanted Defibrillator or Cardiac Resynchronization Therapy (BIO-LIBRA). Heart Rhythm Society 2023 Conference, New Orleans, LA.
2. Kutyifa V, et al. Racial and Sex-Differences in Ventricular Arrhythmias in Patients with Non-Ischemic Cardiomyopathy with Implanted Defibrillators. Heart Rhythm Society 2023 Conference, New Orleans, LA.
About BIOTRONIK
At BIOTRONIK, patient well-being is our top priority and has been for 60 years. BIOTRONIK is a leading global medical technology company with products and services that save and improve the lives of millions suffering from heart and blood vessel diseases as well as chronic pain. Driven by a purpose to perfectly match technology with the human body, we are dedicated innovators who develop trusted cardiovascular, endovascular and neuromodulation solutions. BIOTRONIK is headquartered in Berlin, Germany, and is represented in over 100 countries.