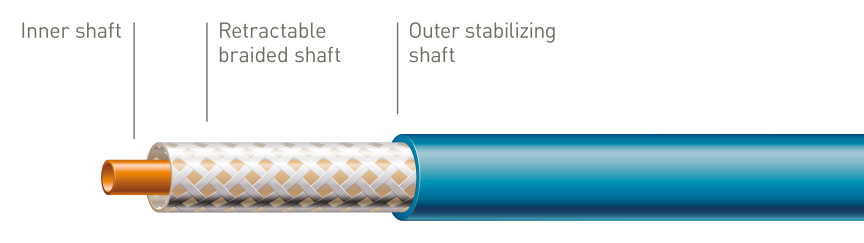
Unique tri-axial shaft design
The outer stabilizing shaft isolates the retractable shaft from friction caused by the introducer valve to ensure accurate stent deployment
Pulsar-18 T3 is indicated for use to improve luminal diameter in patients with symptomatic de novo, restenotic, or occlusive lesions located in the superficial femoral or proximal popliteal arteries, with reference vessel diameters from 3.0 to 6.0 mm and total lesion lengths up to 190 mm.*
The outer stabilizing shaft isolates the retractable shaft from friction caused by the introducer valve to ensure accurate stent deployment

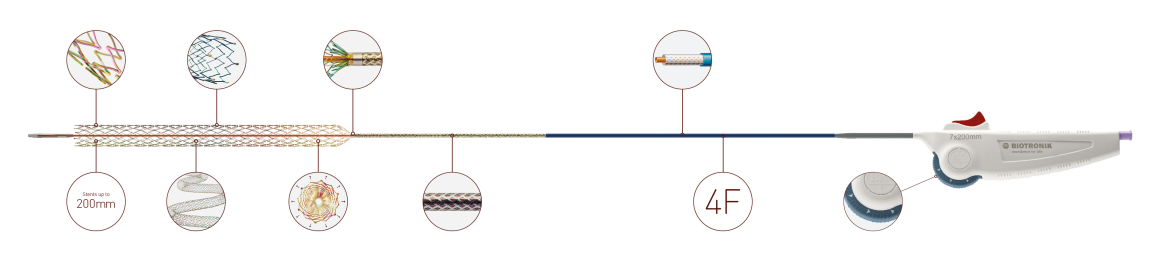
| Stent | |
|---|---|
| Catheter type | OTW |
| Recommended guide wire | 0.018” |
| Stent material | Nitinol |
| Strut thickness | 140 μm |
| Strut width |
85 μm |
| Stent coating | proBIO® (Amorphous Silicon Carbide) |
| Stent markers | 6 gold markers each end |
| Sizes | ø 4.0 - 7.0 mm; L:20 - 200 mm |
| Shaft | 4F, hydrophobic coating, tri-axial |
| Usable length | 90 cm and 135 cm |
| Stent ø (mm) |
Catheter length 90 cm |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20** | 30 | 40 | 60 | 80 | 100 | 120 | 150 | 170 | 200 | ||
| 4F | 4 | 430437 | 430438 | 430439 | 430440 | 430441 | 430442 | 430443 | 430444 | 430445 | 430446 |
| 5 | 430447 | 430448 | 430449 | 430450 | 430451 | 430452 | 430453 | 430454 | 430455 | 430456 | |
| 6 | 430457 | 430458 | 430459 | 430460 | 430461 | 430462 | 430463 | 430464 | 430465 | 430466 | |
| 7 | 430467 | 430468 | 430469 | 430470 | 430471 | 430472 | 430473 | 430474 | 430475 | 430476 | |
| **8 weeks pre-order only | |||||||||||
| Stent ø (mm) |
Catheter length 135 cm |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20** | 30 | 40 | 60 | 80 | 100 | 120 | 150 | 170 | 200 | ||
| 4F | 4 | 430477 | 430478 | 430479 | 430480 | 430481 | 430482 | 430483 | 430484 | 430485 | 430486 |
| 5 | 430487 | 430488 | 430489 | 430490 | 430491 | 430492 | 430493 | 430494 | 430495 | 430496 | |
| 6 | 430497 | 430498 | 430499 | 430500 | 430501 | 430502 | 430503 | 430504 | 430505 | 430506 | |
| 7 | 430507 | 430508 | 430509 | 430510 | 430511 | 430512 | 430513 | 430514 | 430515 | 430516 | |
| **8 weeks pre-order only | |||||||||||
FTLR = Freedom from Target Lesion Revascularization.
1. BIOTRONIK data on file; 2. Zhao HQ. Late stent expansion and neointimal proliferation of oversized nitinol stents in peripheral arteries. Cardiovasc Intervent Radiol. 2009 Jul;32(4):720-6; 3. Bosiers M et al. 4-French – compatible endovascular material is safe & effective in the treatment of femoropopliteal occlusive disease: Results of the 4EVER Trial. ENDOVASC THER 2013; 20: 746-756; 4. Koskinas C. Role of endothelial shear stress in stent restenosis and thrombosis: pathophysiologic mechanisms and implications for clinical translation. JACC 2012 10;59(15):1337-49; 5. Koppara T. Thrombogenicity and early vascular healing response in metallic biodegradable polymer-based and fully bioabsorbable drug-eluting stents. Circ Cardiovasc Interv. 2015 8(6):e002427; 6. Funovics M. Correlation between chronic outward force (COF) and neointimal hyperplasia in self-expanding nitinol stents in swine in clinically relevant oversizing ranges. Presented at: LINC, Jan 26, 2017; Leipzig, Germany, 7. Bosiers M. 4EVER 24 month results: long-term results of 4F Pulsar stent in femoropopliteal lesions. Presented at: CIRSE 2013; Barcelona, Spain; 8. Lichtenberg M. Superficial Femoral Artery TASC D registry: 12-month effectiveness analysis of the Pulsar-18 SE nitinol stent in patients with critical limb ischemia. J Cardiovasc Surg (Torino). 2013 Aug; 54(4):433-9; 9. Lichtenberg et al. Effectiveness of the Pulsar-18 self-expanding stent with optional drug-coated balloon angioplasty in the treatment of femoropopliteal lesions - the BIOFLEX PEACE All-Comers Registry.Vasa (2019), 1-9. doi_10.10240301-1526a000785.
*Indication as per IFU. Pulsar and proBIO are trademarks or registered trademarks of the BIOTRONIK Group of Companies.