Orsiro®Mission
Orsiro Mission DES is indicated for improving coronary luminal diameter in patients, including those with diabetes mellitus, with symptomatic heart disease, stable angina, unstable angina, non-ST elevation myocardial infarction or documented silent ischemia due to atherosclerotic lesions in the native coronary arteries with a reference vessel diameter of 2.25 mm to 4.0 mm and a lesion length of ≤ 36 mm.
Product Highlights
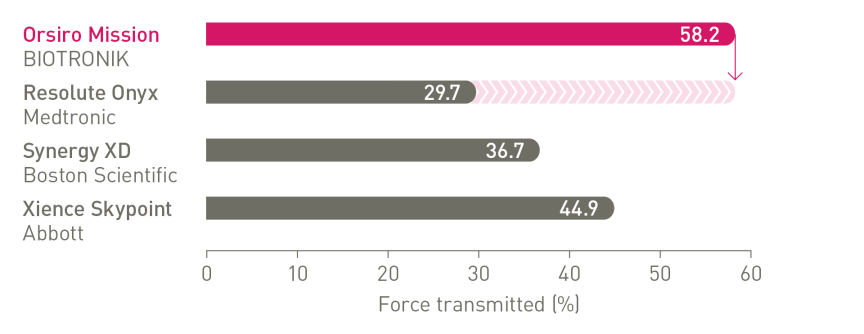
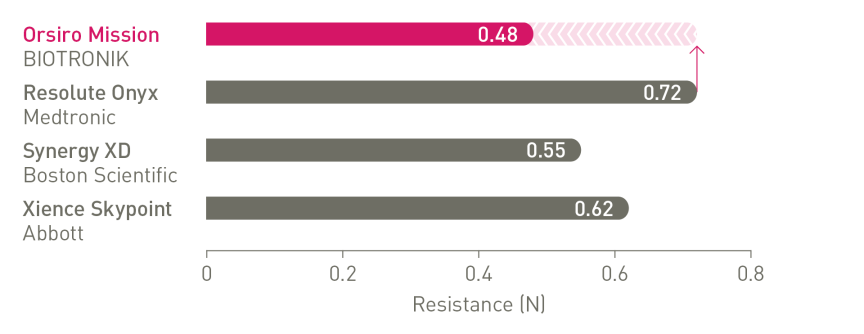
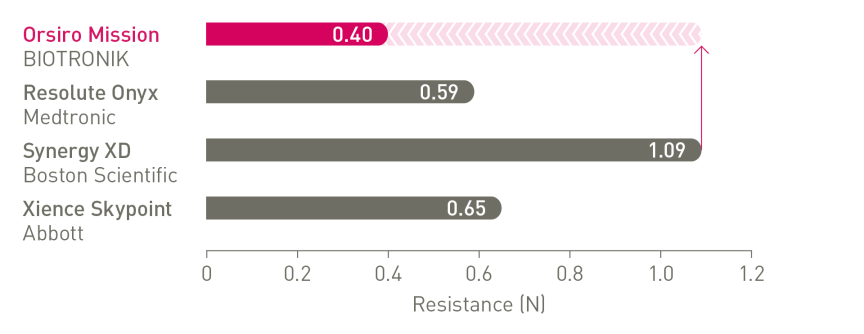
The next level of deliverability¹
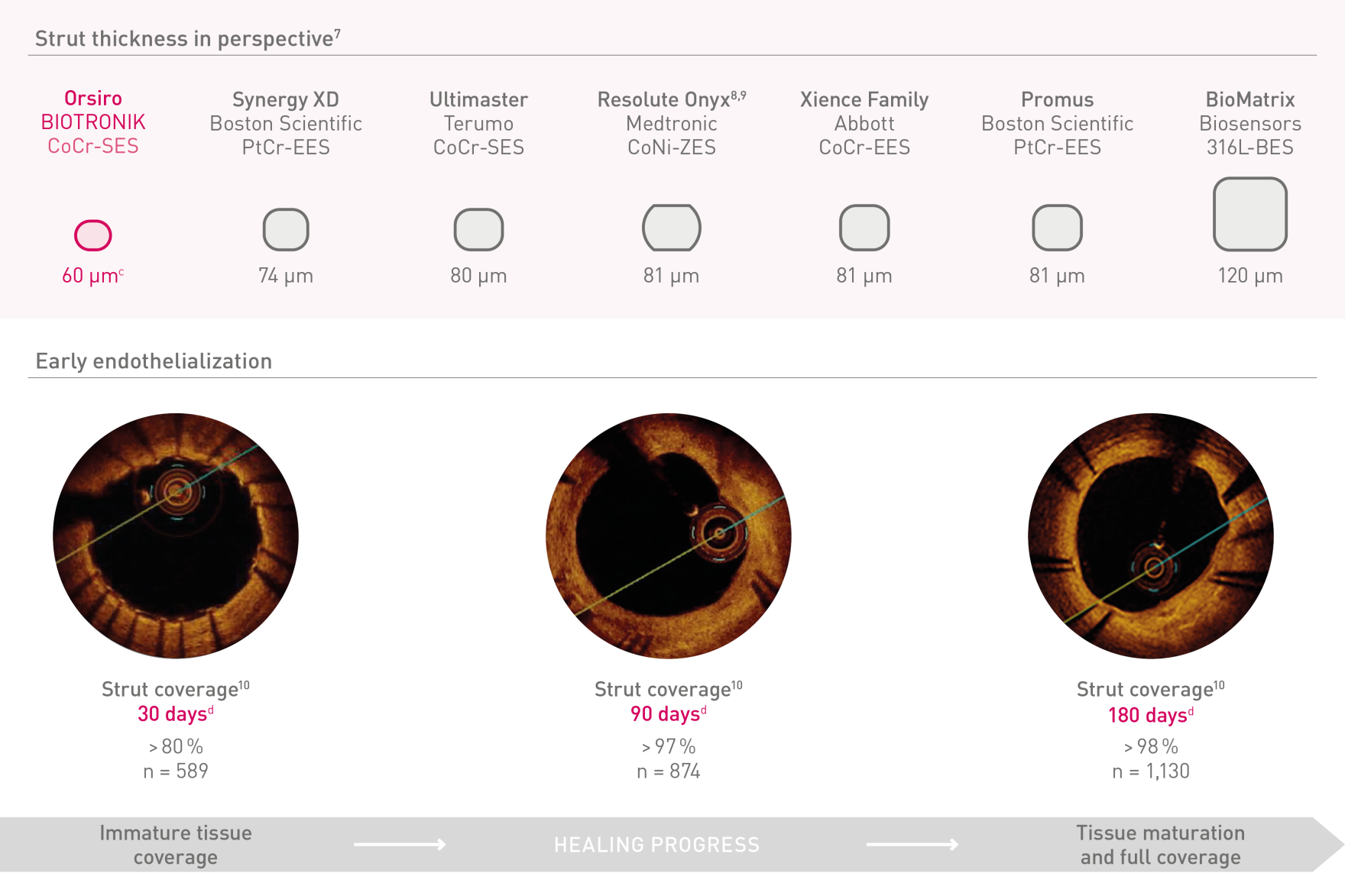
Ultrathin struts²
Outstanding patient outcomes³
Extensive Clinical Program of the Orsiro DES Familyj13
Product Overview
Orsiro Mission
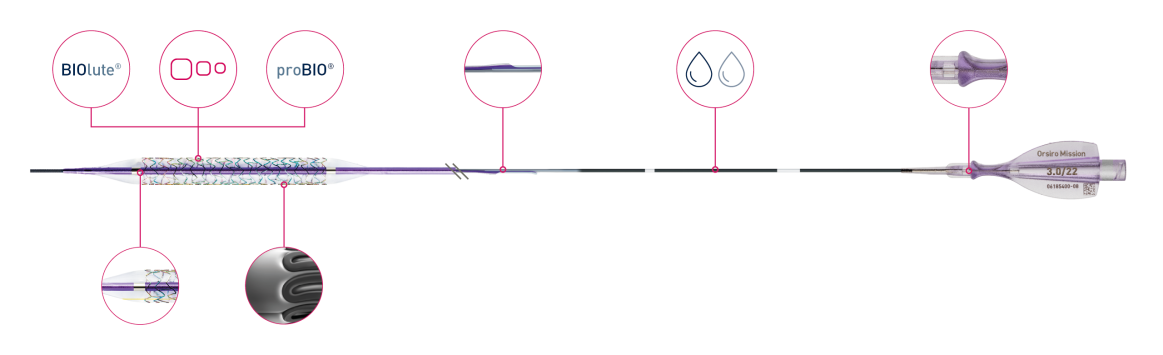
Bioabsorbable coating
Ultrathin 60 µmᶜ struts
Passive coating
Enhanced force transmission
Dual-coating
Ergonomic hub
Flexible shaft
Deep embedding
Technical Data
| Stent | |
|---|---|
| Stent material | Cobalt chromium, L-605 |
| Strut thickness | ø 2.25 – 3.0 mm: 60 µm (0.0024”); ø 3.50 – 4.0 mm: 80 µm (0.0031”) |
| Passive coating | proBIO® (Amorphous Silicon Carbide) |
| Active coating | BIOlute® bioabsorbable Poly-L-Lactide (PLLA) eluting a limus drug |
| Drug dose | 1.4 µg/mm² |
| Delivery system | |
| Catheter type | Fast exchange |
| Recommended guide catheter | 5F (min. I.D. 0.056”) |
| Guidewire diameter | 0.014” |
| Usable catheter length | 140 cm |
| Balloon material | Semi crystalline polymer |
| Coating (Distal shaft) | Hydrophilic |
| Coating (Proximal shaft) | Hydrophobic |
| Marker bands | Two swaged platinum-iridium markers |
| Lesion entry profile | 0.017” |
| Distal shaft diameter | 2.7F: ø 2.25 – 3.0 mm; 2.9F: ø 3.5 – 4.0 mm |
| Proximal shaft diameter | 2.0F |
| Nominal pressure (NP) | 10 atm |
| Rated burst pressure (RBP) | 16 atm |
| Double Helix Stent Design | ||||||
|---|---|---|---|---|---|---|
| Nominal diameter (mm) | 2.25 | 2.5 | 2.75 | 3.0 | 3.5 | 4.0 |
| Strut thickness (μm) | 60 | = | = | = | 80 | = |
| Strut width (μm) | 75 | = | = | = | 85 | = |
| Amount of connectors | 3 | = | = | = | = | = |
| Amount of crowns at end | 8 | = | = | = | = | = |
| Maximal Expansion and Stent Strut Opening | ||||||
|---|---|---|---|---|---|---|
| Nominal diameter (mm) | 2.25 | 2.5 | 2.75 | 3.0 | 3.5 | 4.0 |
| Nominal outer diameter of the stent at NP (mm) | 2.37 | 2.62 | 2.87 | 3.12 | 3.66 | 4.16 |
| Maximal expansion diameter (mm) | 4.0 | = | = | = | 5.0 | = |
| Stent strut opening diameter at NP* (mm) | 0.79 | 0.92 | = | = | 1.06 | 1.25 |
| Maximal diameter of expanded stent cell (mm) | 3.59 | = | = | = | 4.42 | = |
*Mean of the largest possible opening diameter within a stent cell at NP
= symbol used to show repetition
Ordering Information
| Stent ø (mm) |
Stent Length (mm) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 9 | 13 | 15 | 18 | 22 | 26 | 30 | 35 | 40 | |
| 2.25 | 453925 | 453931 | 453937 | 453943 | 453949 | 453955 | 453961 | ||
| 2.5 | 453926 | 453932 | 453938 | 453944 | 453950 | 453956 | 453962 | 453968 | 453974 |
| 2.75 | 453927 | 453933 | 453939 | 453945 | 453951 | 453957 | 453963 | 453969 | 453975 |
| 3.0 | 453928 | 453934 | 453940 | 453946 | 453952 | 453958 | 453964 | 453970 | 453976 |
| 3.5 | 453929 | 453935 | 453941 | 453947 | 453953 | 453959 | 453965 | 453971 | 453977 |
| 4.0 | 453930 | 453936 | 453942 | 453948 | 453954 | 453960 | 453966 | 453972 | 453978 |
Downloads and Related Links
Downloads
Related Links
How can we help you?
References
a. In comparison to Resolute Onyx, BIOTRONIK data on file; b. In comparison to Synergy XD, BIOTRONIK data on file; c. Ø2.25-3.0mm; d. Images: Secco G et al. Time-related changes in neointimal tissue coverage following a new generation SES implantation: an OCT observational study. Presented at: euro PCR, May 20, 2014; Paris, France; e. Bayesian Credible Interval; g. In comparison to Xience, based on statistically significant lower TV-MI and late/very late definite/probable ST rates from the BIOFLOW-V trial through 5 years; h. p-values for 60-month frequentist analysis; i In comparison to Xience, based on BIOFLOW-V 5-year results; j. Orsiro and Orsiro Mission DES; k. All-comers patients, BIO-RESORT 5 year outcomes; l. Based on 1-year TLF SUCRA (Surface Under the Cumulative Ranking Curve), Taglieri Network Meta-Analysis; m.
1. In comparison to Xience Sierra, Resolute Onyx and Synergy for bench tests on pushability, trackability and crossability, BIOTRONIK data on file; 2. As characterized with respect to strut thickness in Bangalore et al. Meta-analysis, Circulation. 2018 Nov 13;138(20):2216-2226; 3. Based on investigator’s interpretation of BIOFLOW-V primary endpoint result; 4. BIOTRONIK data on file; 5. Per investigators’ interpretation in Secco et al. Imaging data serial observations. Secco GG et al. Time-related changes in neointimal tissue coverage of a novel Sirolimus eluting stent: Serial observations with optical coherence tomography. Cardiovascular Revascularization Medicine. 2016; 17(1): 38-43; 7. Stefanini GG et al. Coronary stents: novel developments. Heart. 2014 Jul 1;100(13):1051-61; 8. Low AF. Stent platform for procedural success: Introducing the Continuous Sinusoidal & Core Wire Technologies. Presented at: AsiaPCR; 22-24 January, 2015; Singapore, Singapore; 9. Tolentino A. Evolving DES Strategy: Biodegradable Polymer vs. Bioabsorbable Scaffold. Presented at: Cardiovascular Nurse/Technologist Symposium; June 17, 2016; New York, USA; 10. Secco G et al. Time-related changes in neointimal tissue coverage of a novel Sirolimus eluting stent: Serial observations with optical coherence tomography. Cardiovascular Revascularization Medicine2016; 17(1): 38-43; 7; 12. Kandzari D et al. Ultrathin Bioresorbable Polymer Sirolimus-Eluting Stents Versus Durable Polymer Everolimus-Eluting Stents: BIOFLOW V Final 5-Year Outcomes. JACC Cardiovasc Interv. 2022 Sep 26;15(18):1852-1860; 13. BIOTRONIK data on file, status February 2023; 14. Per investigators’ interpretation of preclinical studies with Orsiro as mentioned in Cassese et al. J Thorac Dis 2018;10(2):688-692; 15. E.Ploumen, BIO-RESORT: 5-year Outcomes from a Randomized Trial of 3 Drug-Eluting Stents in Patients with Coronary Artery Disease, TCT 2021; 16. Taglieri N et al. Target lesion failure with current drug-eluting stents: Evidence from a comprehensive network meta-analysis. JACC 2020 13(24):2868-78.
Clinical data collected with the Orsiro DES device within the Orsiro family clinical program
Orsiro, Orsiro Mission, proBIO and BIOlute are trademarks or registered trademarks of the BIOTRONIK Group of Companies. All other trademarks are the property of their respective owners.