
BIOTRONIK is proud of its 60-year history and innovations
OUR HISTORY
BIOTRONIK From 1963 to Today
BIOTRONIK is proud of its 60-year history and consistent development of life-changing innovations. Our commitment to excellence for life has made us what we are today: a leader in cardiovascular and endovascular medical technology.
Physicist Max Schaldach and electro engineer Otto Franke develop the first German pacemaker at the Technical University of Berlin in 1963 and found BIOTRONIK. In the 1970s, BIOTRONIK opens its headquarters in the Berlin district of Neukölln, where it remains today.

Physicist Max Schaldach and electro engineer Otto Franke develop the first German pacemaker at the Technical University of Berlin in 1963 and found BIOTRONIK. In the 1970s, BIOTRONIK opens its headquarters in the Berlin district of Neukölln, where it remains today.
Due to exceptional technological and commercial success, the company expands throughout Europe and to Asia, North America and South America. BIOTRONIK opens a new production facility in the United States in Lake Oswego, Oregon.

Due to exceptional technological and commercial success, the company expands throughout Europe and to Asia, North America and South America. BIOTRONIK opens a new production facility in the United States in Lake Oswego, Oregon.
BIOTRONIK opens its Brazil office in Sao Paulo, where it remains today. The company further expands its international presence in Europe, South America and Asia.
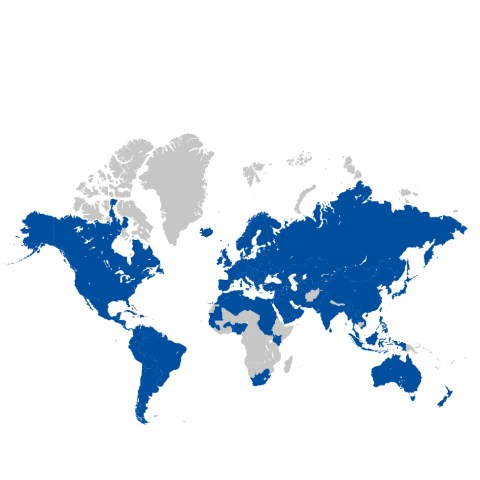
BIOTRONIK opens its Brazil office in Sao Paulo, where it remains today. The company further expands its international presence in Europe, South America and Asia.
BIOTRONIK expands therapy solutions with the first German implantable defibrillator (ICD): Phylax 06, the smallest ICD in the world.
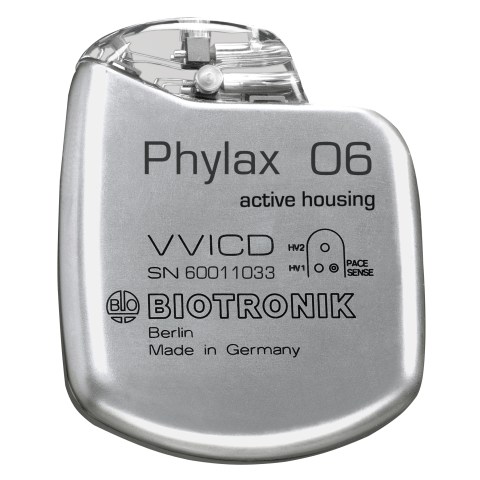
BIOTRONIK expands therapy solutions with the first German implantable defibrillator (ICD): Phylax 06, the smallest ICD in the world.
BIOTRONIK models unique fractal lead coating after nature. Fractal coating creates a snowflake-like pattern that expands the lead’s electrical surface, optimizing its sensing and pacing capabilities.

BIOTRONIK models unique fractal lead coating after nature. Fractal coating creates a snowflake-like pattern that expands the lead’s electrical surface, optimizing its sensing and pacing capabilities.
Another breakthrough: Closed Loop Stimulation, a groundbreaking technology, is the most advanced system to regulate and optimize heart activity based on neurological information. Part of the Inos pacemaker, CLS allows patients’ heart rates to react to physical as well as mental activity and emotion.

Another breakthrough: Closed Loop Stimulation, a groundbreaking technology, is the most advanced system to regulate and optimize heart activity based on neurological information. Part of the Inos pacemaker, CLS allows patients’ heart rates to react to physical as well as mental activity and emotion.
Business operations expand to include vascular intervention. BIOTRONIK opens a site in the Swiss town of Bülach, outside Zurich, which manufactures guide wires, stents and balloon catheters for the treatment of cardiovascular and endovascular disease.

Business operations expand to include vascular intervention. BIOTRONIK opens a site in the Swiss town of Bülach, outside Zurich, which manufactures guide wires, stents and balloon catheters for the treatment of cardiovascular and endovascular disease.
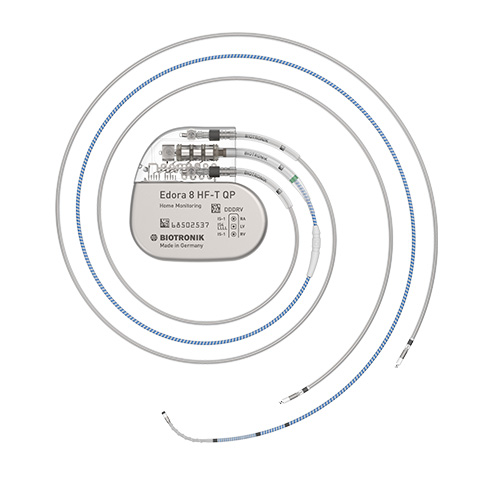
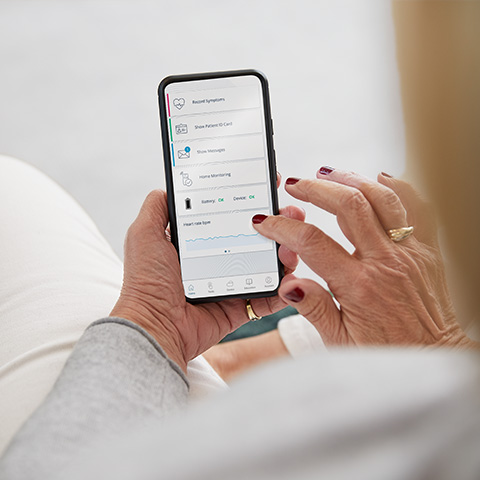
The company focuses on diagnostic and sensor technology. With the development of BIOTRONIK Home Monitoring®, we pioneer the ability to monitor patients outside the physician’s office, enabling early detection and treatment of critical events such as cardiac arrhythmias.
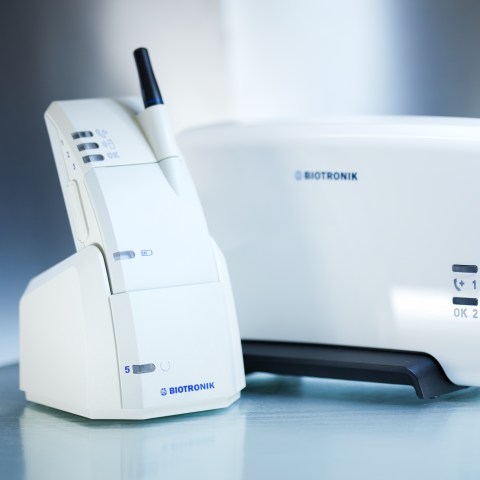
The company focuses on diagnostic and sensor technology. With the development of BIOTRONIK Home Monitoring®, we pioneer the ability to monitor patients outside the physician’s office, enabling early detection and treatment of critical events such as cardiac arrhythmias.
Therapy solutions expand to include pacemakers with cardiac resynchronization therapy (CRT). These are the first worldwide to offer remote monitoring.
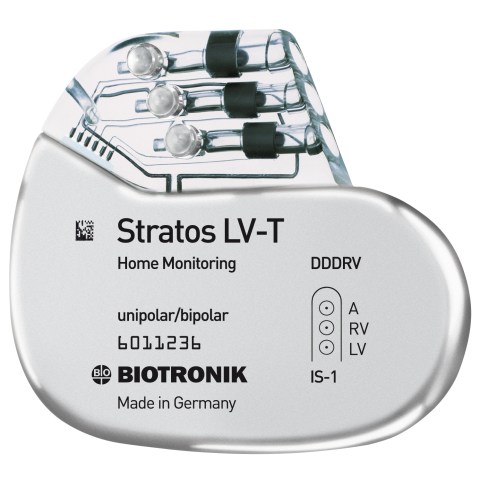
Therapy solutions expand to include pacemakers with cardiac resynchronization therapy (CRT). These are the first worldwide to offer remote monitoring.
Innovation in the field of electrophysiology: for the effective and safe treatment of patients with atrial fibrillation, BIOTRONIK launches AlCath Gold, the first ablation catheter with a gold tip.

Innovation in the field of electrophysiology: for the effective and safe treatment of patients with atrial fibrillation, BIOTRONIK launches AlCath Gold, the first ablation catheter with a gold tip.
BIOTRONIK launches the Pantera coronary balloon to treat patients suffering from coronary artery disease.

BIOTRONIK launches the Pantera coronary balloon to treat patients suffering from coronary artery disease.
BIOTRONIK’s Evia pacemakers offer patients the unique combination of small size and exceptional battery life that lasts more than a decade.

BIOTRONIK’s Evia pacemakers offer patients the unique combination of small size and exceptional battery life that lasts more than a decade.
BIOTRONIK pacemakers include ProMRI® technology for the first time, enabling patients to safely undergo MRI scans.
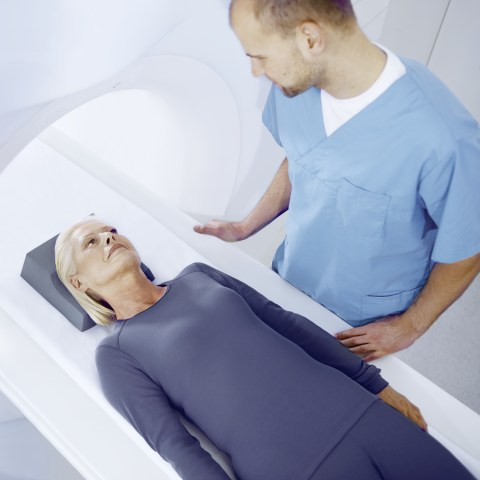
BIOTRONIK pacemakers include ProMRI® technology for the first time, enabling patients to safely undergo MRI scans.
The Lumax 540 VR-T DX implantable defibrillator comes with another innovation: the world’s first single-chamber ICD with complete atrial diagnostics.

The Lumax 540 VR-T DX implantable defibrillator comes with another innovation: the world’s first single-chamber ICD with complete atrial diagnostics.
With Pulsar-18*, the world’s first 4 French-compatible self-expanding stent, BIOTRONIK sets new standards in minimally invasive treatment of peripheral artery disease.

With Pulsar-18*, the world’s first 4 French-compatible self-expanding stent, BIOTRONIK sets new standards in minimally invasive treatment of peripheral artery disease.
BIOTRONIK launches the drug-eluting balloon Pantera Lux* to treat the re-narrowing of a coronary artery.

BIOTRONIK launches the drug-eluting balloon Pantera Lux* to treat the re-narrowing of a coronary artery.
With Orsiro*, BIOTRONIK offers the world’s first hybrid drug-eluting stent with a bioabsorbable coating. With its unique design and exceptional deliverability, Orsiro marks a new era in the treatment of coronary artery disease.

With Orsiro*, BIOTRONIK offers the world’s first hybrid drug-eluting stent with a bioabsorbable coating. With its unique design and exceptional deliverability, Orsiro marks a new era in the treatment of coronary artery disease.
BIOTRONIK strengthens its engagement in Asia, opening an office in Singapore to direct sales and marketing in the region.

BIOTRONIK strengthens its engagement in Asia, opening an office in Singapore to direct sales and marketing in the region.
With Lumax 740 ProMRI®, BIOTRONIK brings the world’s first ICD to the market that enables patients to safely access MRI scans.

With Lumax 740 ProMRI®, BIOTRONIK brings the world’s first ICD to the market that enables patients to safely access MRI scans.
BIOTRONIK launches BioMonitor – an implantable cardiac monitor – with ProMRI technology to reliably monitor patients with cardiac rhythm disorders long-term, while enabling access to MRI scans.

BIOTRONIK launches BioMonitor – an implantable cardiac monitor – with ProMRI technology to reliably monitor patients with cardiac rhythm disorders long-term, while enabling access to MRI scans.
The company unveils the PK Papyrus* covered single stent system to treat acute perforations in the coronary artery.
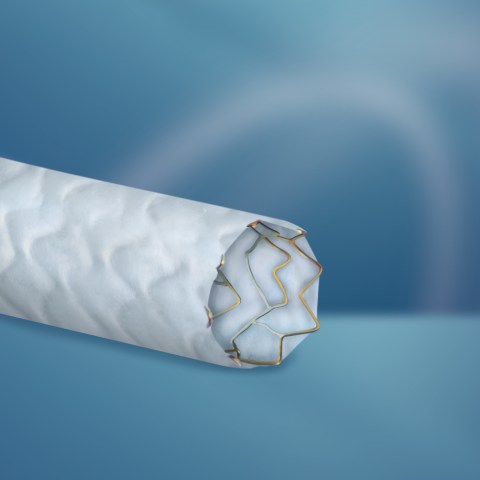
The company unveils the PK Papyrus* covered single stent system to treat acute perforations in the coronary artery.
The drug-coated balloon Passeo-18 Lux* is indicated to treat peripheral artery disease in the lower limbs.

The drug-coated balloon Passeo-18 Lux* is indicated to treat peripheral artery disease in the lower limbs.
CardioMessenger Smart, the newest patient device for Home Monitoring, is the size of a smart phone. The device transmits data from a patient’s pacemaker or ICD to their physician, enabling patient monitoring anytime, anywhere.

CardioMessenger Smart, the newest patient device for Home Monitoring, is the size of a smart phone. The device transmits data from a patient’s pacemaker or ICD to their physician, enabling patient monitoring anytime, anywhere.
Magmaris is the first resorbable magnesium scaffold on the market indicated for the treatment of de novo coronary artery lesions. The device provides novel benefits that only a magnesium scaffold can offer like fast resorption, compelling safety data and better deliverability.
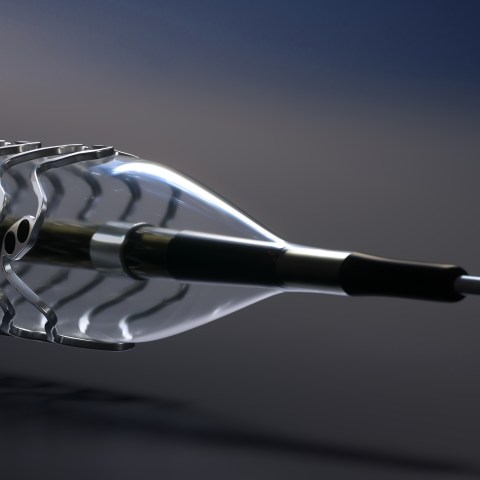
Magmaris is the first resorbable magnesium scaffold on the market indicated for the treatment of de novo coronary artery lesions. The device provides novel benefits that only a magnesium scaffold can offer like fast resorption, compelling safety data and better deliverability.




Physicist Max Schaldach and electro engineer Otto Franke develop the first German pacemaker at the Technical University of Berlin in 1963 and found BIOTRONIK. In the 1970s, BIOTRONIK opens its headquarters in the Berlin district of Neukölln, where it remains today.

Due to exceptional technological and commercial success, the company expands throughout Europe and to Asia, North America and South America. BIOTRONIK opens a new production facility in the United States in Lake Oswego, Oregon.

BIOTRONIK opens its Brazil office in Sao Paulo, where it remains today. The company further expands its international presence in Europe, South America and Asia.

BIOTRONIK expands therapy solutions with the first German implantable defibrillator (ICD): Phylax 06, the smallest ICD in the world.

BIOTRONIK models unique fractal lead coating after nature. Fractal coating creates a snowflake-like pattern that expands the lead’s electrical surface, optimizing its sensing and pacing capabilities.

Another breakthrough: Closed Loop Stimulation, a groundbreaking technology, is the most advanced system to regulate and optimize heart activity based on neurological information. Part of the Inos pacemaker, CLS allows patients’ heart rates to react to physical as well as mental activity and emotion.

Business operations expand to include vascular intervention. BIOTRONIK opens a site in the Swiss town of Bülach, outside Zurich, which manufactures guide wires, stents and balloon catheters for the treatment of cardiovascular and endovascular disease.

The company focuses on diagnostic and sensor technology. With the development of BIOTRONIK Home Monitoring®, we pioneer the ability to monitor patients outside the physician’s office, enabling early detection and treatment of critical events such as cardiac arrhythmias.

Therapy solutions expand to include pacemakers with cardiac resynchronization therapy (CRT). These are the first worldwide to offer remote monitoring.

Innovation in the field of electrophysiology: for the effective and safe treatment of patients with atrial fibrillation, BIOTRONIK launches AlCath Gold, the first ablation catheter with a gold tip.

BIOTRONIK launches the Pantera coronary balloon to treat patients suffering from coronary artery disease.

BIOTRONIK’s Evia pacemakers offer patients the unique combination of small size and exceptional battery life that lasts more than a decade.

BIOTRONIK pacemakers include ProMRI® technology for the first time, enabling patients to safely undergo MRI scans.

The Lumax 540 VR-T DX implantable defibrillator comes with another innovation: the world’s first single-chamber ICD with complete atrial diagnostics.

With Pulsar-18*, the world’s first 4 French-compatible self-expanding stent, BIOTRONIK sets new standards in minimally invasive treatment of peripheral artery disease.

BIOTRONIK launches the drug-eluting balloon Pantera Lux* to treat the re-narrowing of a coronary artery.

With Orsiro*, BIOTRONIK offers the world’s first hybrid drug-eluting stent with a bioabsorbable coating. With its unique design and exceptional deliverability, Orsiro marks a new era in the treatment of coronary artery disease.

BIOTRONIK strengthens its engagement in Asia, opening an office in Singapore to direct sales and marketing in the region.

With Lumax 740 ProMRI®, BIOTRONIK brings the world’s first ICD to the market that enables patients to safely access MRI scans.

BIOTRONIK launches BioMonitor – an implantable cardiac monitor – with ProMRI technology to reliably monitor patients with cardiac rhythm disorders long-term, while enabling access to MRI scans.

The company unveils the PK Papyrus* covered single stent system to treat acute perforations in the coronary artery.

The drug-coated balloon Passeo-18 Lux* is indicated to treat peripheral artery disease in the lower limbs.

CardioMessenger Smart, the newest patient device for Home Monitoring, is the size of a smart phone. The device transmits data from a patient’s pacemaker or ICD to their physician, enabling patient monitoring anytime, anywhere.

Magmaris is the first resorbable magnesium scaffold on the market indicated for the treatment of de novo coronary artery lesions. The device provides novel benefits that only a magnesium scaffold can offer like fast resorption, compelling safety data and better deliverability.






