Key Results
Key Result 1
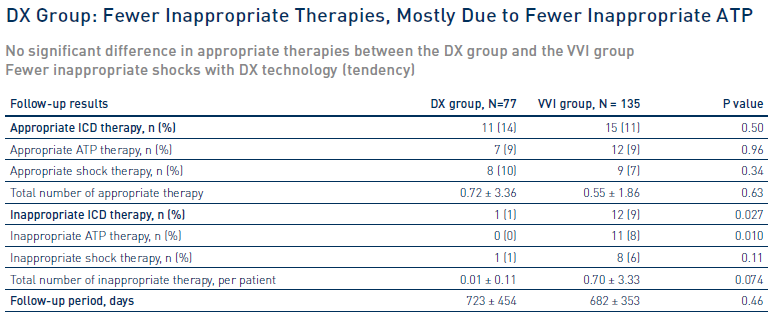
Significantly fewer inappropriate ICD therapies with DX technology


The incidence of inappropriate ICD therapy was lower with DX, even though a previous history of AF was more frequent in the DX group.
Avoiding inappropriate therapy of single‐lead implantable cardioverter‐defibrillator by using atrial‐sensing electrodes
Kurt M et al., Journal of Cardiovascular Electrophysiology, September 2018. doi: 10.1111/jce.13736
Investigator-initiated trial, not funded by BIOTRONIK


The incidence of inappropriate ICD therapy was lower with DX, even though a previous history of AF was more frequent in the DX group.
No significant differences in fluoroscopic burden and operative duration between in th DX group and the VVI group

Consecutive patients undergoing primary prophylactic single-lead ICD implantation or replacement at the institute in the context of Düsseldorf University Device Registry
ICD replacement: patients with single-lead ICD and no previously documented ventricular tachycardia/ventricular fibrillation (VT/VF) or ICD therapies including antitachycardia pacing (ATP) and shock
Indication for ICD: based on classes I and IIa recommendations from the 2015 European Society of Cardiology Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death1
DX-ICD implantation: Lumax VR-T DX, Iforia VR-T DX, or Itrevia VR-T DX device, combined with Linox Smart S DX or Linox Smart ProMRI S DX lead
VVI-ICD implantation: selection of device at implanter’s discretion
1 Priori SG, Blomström‐Lundqvist C, Mazzanti A, et al. Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace. 2015;17:1601‐1687